valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Lead(II) and Manganese(II) formate syntheses
Hello everyone! This is my first synthesis on sciencemadness after many years of inactivity. Any further insights on these compounds will be very
welcome, as I've found them to be quite minor and irrelevant to the modern chemical industry, and little to no information is found online. I'm
including some excerpts from a couple of 19th-century books from my library.
These salts are synthesized with the same method, changing only the stoichiometry. That's why the title is "Lead(II) and Manganese(II) formate".
--Lead(II) Formate--
Having recently bought a litre of 80% formic acid from a friend, I decided I'd try the synthesis of a trivial lead(II) salt: lead(II) formate. This
synthesis can reportedly be carried out with formic acid of any concentration, any lead oxide (minium, litharge o dioxide -called "flea oxide" in
Italian because of its dark brown colour) and changing the amount of water to taste.
This salt is fairly useful and cheap to make if you need to convert the relatively useless lead(IV) oxide to a lead(II) salt in an inexpensive and
safe way. This allows you to process and make use of waste lead oxide without dealing with strong acids, peroxide, chlorine and other gases, and
reducing the splattering and boiling of solutions to almost zero.
I'm using battery-grade lead dioxide. My reaction is:
PbO2 + 3HCOOH -> Pb(CHOO)2 + CO2 + 2H2O (remember to adjust the stoichiometry when changing oxides)
First run:
I used 0.76g of PbO2 (0.0031mol), 11.1mL of HCOOH 80% (excess reagent) and 60mL of water. Placed in a 100mL Erlenmeyer flask and lightly
heated, the reactants quickly disappear and the solution fades from a turbid brown to a colourless one in a matter of 5 minutes. Filtered twice on
cotton, crystallized in a homemade peltier freezing chamber, evaporated to half its volume and cooled again, it yielded two batches of perfectly white
crystals, briefly washed with acetone and dried for a week over NaOH. The first batch yielded needle-like white crystals, while the second batch
resembled a little cotton ball. Total yield of 0.74g on a theoretical of 0.943g, 77.3%.
Second run:
I used 0.89g of PbO2 (0.0037mol), 10.0mL of HCOOH 80% (excess reagent) and 20mL of water. Filtered thrice on cotton (volume was 20mL after
filtering), crystallized in a homemade peltier freezing chamber, evaporated to half its volume and cooled again, it yielded two batches of perfectly
white crystals, briefly washed with acetone and dried for a week over NaOH. The first batch has needle-like crystals, much better than in the first
run, while the second batch has even more fibrous-looking ones. Total yield of 0.65g on a theoretical 1.094g, 59.3%.
The yields could be very well increased by crystallizing further, but I stopped at the second one, happy with my 1.39g of salt. The compound MUST be
dried over NaOH or KOH to fully remove HCOOH vapours and traces of acid, especially if there are other substances in the desiccator. The drying is
complete when the salt looks and feels dry (duh!) and doesn't smell of formic acid anymore, though I'd be careful smelling such minute Pb(II)
crystals. The obtained product is anhydrous, and its solubility is around 2.06g/100mL at 27.5°C (titration procedure is here by another member on another forum, in Italian. It's not my work so I'm not going to copy-paste it, but it involves xylenol orange,
hexamethylentetrammine and EDTA).
Now. For anyone with a home lab and the epic struggle with battery-grade MnO2, a most nasty, useless and stupid chemical whose only role
seems to be that of laughing at you from the shelves and showing off its huge carbon content, the next question might be enlightening. Can we make
Manganese(II) formate in the same clean, quick and cheap way? YES.
--Manganese(II) formate dihydrate--
As for with lead, you can use any formic acid and any amount of water. The only thing that's going to be affected is most likely the yield and the
amount of power to evaporate the solution.
The reaction is (assuming the salt is anhydrous for ease):
MnO2 + 3HCOOH -> Mn(CHOO)2 + CO2 + 2H2O
I used 6.98g of well-washed and dried battery-grade MnO2 (assuming, in the worst case, a 100% content of dioxide, 0.079mol), 20mL of HCOOH
80% (0.347mol, excess reagent) and 20mL of water. Placed the chemicals in a 100-mL Erlenmeyer, a quick and vigorous but safe bubbling is observed, and
slowly ceases after around 10 minutes. The solution is filtered twice on cotton, to remove any carbon traces, evaporated to 20mL and crystallized in a
homemade Peltier freezing cell.
With the first filtration it becomes evident the solution has a very light yellowish colour that quickly turns into a deep brown orange shade after
evaporating to 20mL. This solution is quickly crystallized at around -5°C with the aid of a few mL of 93% EtOH yielding a batch of fine needle-like
crystals of a most amazing apricot-orange shade. After two weeks of drying over NaOH they turn out to be pearlescent, fine and almost amorphous
custard/apricot coloured shavings (weird!), with a feeble scent of ethyl formate. Mother liquors are evaporated to dryness and thus yield a mass of
bulky wine-red crystals.
the first batch yielded 4.40g of apricot-coloured crystals, the second batch 2.11g of wine-red ones. Total yield is 6.51g.
Desiccation hints are the same as for lead formate. Both batches are positive for Mn(II) with S2- and negative for Fe(II) and Fe(III) with
S2- and SCN-.
We can now discuss these results, and draw some conclusions.
Having detected a huge amount of unreacted black matter despite the huge excess of formic acid, we can happily reject the hypothesis of our
dioxide being pure (yield would have been 11.64g of anhydrous salt or 14.53g dihydrate).
If we estimate the actual yield to be around 80% (like with lead) and the product to be fully anhydrous, much unlikely, (mw= 144.97) our dioxide
would be no more than 69.9% MnO2.
If we estimate the actual yield to be around 80% (like with lead) and the product to be fully dihydrate (mw= 180.97) our dioxide would be no
more than 56.0% MnO2.
The mother liquors have been fully dried, so the yield is *most likely* more than 80%, but I'd rather be cautious with that.
These conclusions are obviously not analytically relevant, but can be used to pinpoint an estimated % of the purity of an impure lead or manganese
dioxide (in my case it is at least 51% manganese dioxide). Determination of manganese dioxide in battery-grade pyrolusite can be done with a return
titration by excess oxalate with permanganate (found on the Sutcliffe, Practical Chemistry for Advanced Students 1949, p. 56 exp. 67, p. 83 exp. 103).
--Notes and External Resources--
As for the lead salt, there are little to no additional information on the Woollins, Inorganic Experiments of 2010. The yield is reported to be around
80%. On the link above tot he Italian forum, another user claims the Kps of Pb(CHOO)2 to be 2*10-7 at 25 °C (no further source
provided).
Lead formate was employed as an intermediate in an old, obsolete synthetical process for HCOOH. Equal weigths of glycerine and oxalic acid are heated
to 212-220 F for 15 hours. The product is distilled with lots of water, glycerin is regenerated and formic acid passes over, is concentrated and
removed with lead carbonate. The lead formate is decomposed under hydrogen sulfide, the syrupy acid collected, concentrated, purified and
sold.(Chemistry: General, Medical, and Pharmaceutical by John Attfield, 1889). Another method employed the boiling of starch, manganese dioxide and
diluted sulphuric acid, the saturation with lead carbonate of the obtained liquid and as above. On lead formate it just says "produces long, needly
crystals of no colour, a little soluble in cold water" (Chimica Moderna by Adolphe Wurtz, 1877). The Chimie Médicale by Wurtz has only a deeper
insight on the second sythetical process (the one with starch) but doesn't give us any more infos. I have not found any information regarding these
salts in the Pharmacopoeia Britannica of 1928 and 1846.
The Chemistry of Manganese, Technetium and Rhenium by Kemmitt, Peacock (pages 821-822) tells us that manganese formate is a dihydrate salt forming
monoclinic red crystals; other sources claim you can obtain an anhydrous formate by re-crystallizing it from hot formic acid and that it decomposes to
CO and MnO at around 315°C.
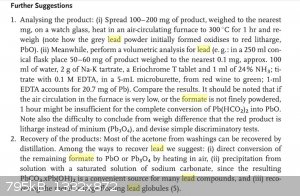
--Photos--
Photos depict lead(II) formate according to run.batch numbers, and manganese formate. I'm also including a screenshot from the Woollins' free preview
on Google Books briefly dealing with lead formate analysis.
    
Have a nice day!
[Edited on 9-9-2017 by valeg96]
[Edited on 10-9-2017 by valeg96]
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
valeg96,
A very nice write up. Hope to see more.
Also, thank you for the link to the Italian chemistry site which is quite interesting. Now I can practice my Italian while reading about chemistry. Is
the home/amateur chemistry hobby big in Italy?
Currently I am in Tuscany, enjoying the food and wine and lots of rain. What happened to the drought?
AvB
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by AvBaeyer  | Is the home/amateur chemistry hobby big in Italy?
Currently I am in Tuscany, enjoying the food and wine and lots of rain. What happened to the drought? |
Hey! Enjoy Tuscany, it's a beautiful region, too warm for me but beautiful indeed. Droughts are pretty frequent in some regions, especially where bad
maintenance causes huge losses of water from municipal pipes... but that's not a topic I know enough about. Forest fires are another spectacular
example of Italian summer 
About the hobby, it's somehow big, but there are no platforms for the participants to meet, that forum itself is ridden with stuck-up people that
"insult away" any beginner, so it's definitely not in good shape, but things are going to change soon. Glad you liked this synthesis; I still have to
figure out how Prepublication works... should I share this over there in the future?
[Edited on 10-9-2017 by valeg96]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Hi valeg96,
Great write-up, thanks. It's also nice to read about simple compounds that no-one usually speaks about.
Quote: Originally posted by valeg96  | (...)
With the first filtration it becomes evident the solution has a very light yellowish colour that quickly turns into a deep brown orange shade after
evaporating to 20mL. This solution is quickly crystallized at around -5°C with the aid of a few mL of 93% EtOH yielding a batch of fine needle-like
crystals of a most amazing apricot-orange shade. After two weeks of drying over NaOH they turn out to be pearlescent, fine and almost amorphous
custard/apricot coloured shavings (weird!), with a feeble scent of ethyl formate. Mother liquors are evaporated to dryness and thus yield a mass of
bulky wine-red crystals.
(...)
[Edited on 10-9-2017 by valeg96] |
I wouldn't exclude that the yellowish crystals are the anhydrate, and the dark brown ones the dihydrate. For many compounds, the hydrates are much
darker or more strongly coloured than the anhydrates.
Did you weight the undissolved matter from the Mn(HCOO)2 synthesis? This would give you a good hint about the purity of your starting
material. I own a vial of technical grade MnO2, purchased a few decades ago at a pharmacy store. I checked the purity a few years back, and
(to my astonishment) it only was around 85%, the main impurity being iron.
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by Bezaleel  | Hi valeg96,
Great write-up, thanks. It's also nice to read about simple compounds that no-one usually speaks about.
I wouldn't exclude that the yellowish crystals are the anhydrate, and the dark brown ones the dihydrate. For many compounds, the hydrates are much
darker or more strongly coloured than the anhydrates.
Did you weight the undissolved matter from the Mn(HCOO)2 synthesis? This would give you a good hint about the purity of your starting
material. |
Thank you. Knowing your lab work is useful and appreciated by others is probably the most rewarding thing in amateur chemistry.
About the undissolved matter, my plan was originally that of collecting everything, estimating the purity and running the thing again to check whether
there was still dioxide, but the carbon was SO fine and nasty that it was basically impossible to fully recover from the cotton and the glassware, to
such an extent that it wouldn't've been accurate. So I just assumed everything reacted and gave up on that.
I'm intrigued by the suggestion that the yellow batch could be the anhydrous salt, but I am not really a fan (or too chemically equipped either) of
transition-metal titrations, so I don't know how to check that. It's reasonable, the crystals barely look cristalline (more like pearlescent amorphous
scales). I honestly thought the colour difference was caused by the particle size difference in the products, but your suggestion seems way more
realistic.
About your sample I guess I've been lucky, as there's no appreciable iron in my dioxide. 15% of mainly Fe impurities is absurd! Technical grade, at a
pharmacy? Wouldn't call it a rip-off but it's surely not a fair sale.
Thank you again for your insights!
[Edited on 11-9-2017 by valeg96]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by valeg96  | | About the undissolved matter, my plan was originally that of collecting everything, estimating the purity and running the thing again to check whether
there was still dioxide, but the carbon was SO fine and nasty that it was basically impossible to fully recover from the cotton and the glassware, to
such an extent that it wouldn't've been accurate. So I just assumed everything reacted and gave up on that. |
I should have known, had the same problem with carbon contamination.
Quote: Originally posted by valeg96  | | I'm intrigued by the suggestion that the yellow batch could be the anhydrous salt, but I am not really a fan (or too chemically equipped either) of
transition-metal titrations, so I don't know how to check that. It's reasonable, the crystals barely look cristalline (more like pearlescent amorphous
scales). I honestly thought the colour difference was caused by the particle size difference in the products, but your suggestion seems way more
realistic. |
Both may be true. Difference in particle size can introduce surprisingly big changes of colour
too.
You could try to heat some of the dark red crystals (stay far enough below the Mn(HCOO)2 decomposition point) and see what you get. If they
effloresce and yield the same yellow stuff, I guess you can be pretty sure the yellow stuff has no crystal water.
Quote: Originally posted by valeg96  | | About your sample I guess I've been lucky, as there's no appreciable iron in my dioxide. 15% of mainly Fe impurities is absurd! Technical grade, at a
pharmacy? Wouldn't call it a rip-off but it's surely not a fair sale. |
A bit of background - when I purchased
it, I was asked what I would use it for. Since it was only intended as a catalyst for the decomposition of hydrogen peroxide, he sold me the cheaper
stuff. I didn't understand what it was all about then. It was one of the very first experiments I ever did. That's why I remember it so well, I guess.
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by Bezaleel  |
Both may be true. Difference in particle size can introduce surprisingly big changes of colour too.
You could try to heat some of the dark red crystals (stay far enough below the Mn(HCOO)2 decomposition point) and see what you get. If they
effloresce and yield the same yellow stuff, I guess you can be pretty sure the yellow stuff has no crystal water.
|
Thanks, will do, and have the first post updated. For some reason it's not editable anymore. I might also try making Sn(II), Cu(II) and Fe(II) salts,
I sort of got into formates after all the research behind this double synthesis...
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
With formates, I would be exploring some radical based chemistry with the reducing .CO2- radical anion.
For example, a paper, "Highly reductive radical .CO2− deriving from a system with .SO4− and formate anion: Implication for reduction of Cr(VI)
from wastewater", at http://www.sciencedirect.com/science/article/pii/S1385894716... .
Interestingly, ultraviolet (VUV, 172 nm) irradiation of aqueous solution containing formate forms the .CO2- radical anion. See http://onlinelibrary.wiley.com/doi/10.1002/poc.365/epdf?r3_r...
The action of the hydroxyl radical, however produced, on the formate anion creates the .CO2- reducing radical:
HCO2- + .OH = H2O + .CO2-
[Edited on 13-9-2017 by AJKOER]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Thank you for this new information. I read the first paper only (for some reason my institution doesn't include Wiley in their subscriptions) and it
seems quite a curious reaction I'd like to try. Looks like a rather straightforward one: I just need to get a hold of some persulfate.
Regarding the second article, I don't really have many organic compounds, so I assume I couldn't replicate anything in there. I've honestly never
heard of the CO2.- and the SO4.- radicals, but I'll study this topic more. I'm also planning to synthesise
a wide variety of single and double formates, in the near future. Thank you!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
See below, perhaps your solution oxidized in air. In looking up Mn(II) Acetate, it wouldn't make sense for you to obtain an Mn(II) salt that's not a
very light pink...also, it would make even less sense that somehow your product was something reduced from Mn(II) as AJOKER Suggests. I suggest
dissolving and doing more tests to determine which Mn oxidation state(s) you have.
Good luck! Certainly cool to see a pretty product from that ugly MnO2...
Quote: Originally posted by Amos  | Quote: Originally posted by Brain&Force  | Could it be manganese(III) chloride?
No Tears Only Dreams Now had a pic of it but I couldn't remember if it was purple or brown. |
Here's the picture in question. I obtained that solution, which has been filtered no less than 4 times, by treating pottery grade manganese dioxide
with boiling hydrochloric acid under reflux for a couple of hours. Searching for "manganese(III)" gives pictures of amber or brown colored solutions
just like mine, though the coloration could also be due to impurities of iron(III) chloride. I'm not entirely sure that MnCl3 exists under normal
conditions. Treating my brown solution(which contained remaining hydrochloric acid) with sodium metabisulfite made it give way to a pale yellow
solution, which might be manganese(II) chloride with some residual iron.
|
Click on the link by Amos' name to see the photo he posts.
Also, see ChemPlayer on Mn(III) acetate and its color:
https://www.youtube.com/watch?v=Q_fSFtLYS1I
[Edited on 9-14-2017 by The Volatile Chemist]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by The Volatile Chemist  | See below, perhaps your solution oxidized in air. In looking up Mn(II) Acetate, it wouldn't make sense for you to obtain an Mn(II) salt that's not a
very light pink...also, it would make even less sense that somehow your product was something reduced from Mn(II) as AJOKER Suggests. I suggest
dissolving and doing more tests to determine which Mn oxidation state(s) you have.
Good luck! Certainly cool to see a pretty product from that ugly MnO2... |
I highly doubt it's a Mn(III) salt, as literature (quoted in my post and below here) says Mn(II) formate dihydrate is a wine-red solid, as I got. And
I assume the pale orange one is an anhydrous form, or a very fine dihydrate. Your hypothesis seems improbable, as it's relatively easy to oxidize
formic acid to CO2 and the Mn(III) would most likely be reduced back to Mn(II)in such conditions of excess acid:
E0CO2/formate=-0.42V and E0Mn(III)/Mn(II)=1.5V. It seems unlikely that I could have isolated such an
oxidizing 3+ ion with conditions so bland and polar: The Chemistry of Mn, Tc, Rh by Kemmitt-Peacock says that Mn(III) formate is yes, a red solid that
can be formed by reacting the dioxide in formic acid (other sources also suggest formic acid in permanaganate), but it also says it quickly
disproportionates to Mn(II) and Mn(IV) with water or ethanol, and I have used both of them, and they had their chance to react while boiling and
everything, and the acid was only at 80%. Also, the reaction was basically carried out under refluxing vapours of formic acid, with a watchglass
keepkng the vessel relatively closed, so not exactly too exposed to oxygen. Also, not all manganese compounds are necessarily light pink or purple,
and that's not a very reliable criterium. Not all copper compounds are blue and not all nickel compounds are green. Don't be misled by "classical"
colours of cations.
Sources: Manganese(III) reduction potentials
Formic acid reduction potential
Chemistry of Mn, Tc, Rh Kemmitt-Peacock
[Edited on 14-9-2017 by valeg96]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Cool, ok, sounds good. Thanks for providing the sources. I was just surprised at the color. If your procedure indeed produces a clean, crystalline
Mn(II) salt as it appears (from MnOx/C junk), then this is certainly something that I should look into. Good work! (Also, the compounds
you've made certainly are pretty - always nice when they're colorful for a change!)
-TVC, Nathan
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
No, thanks to you, Nathan. The questions and doubts you raised incited me to do some research, and learn some additional facts, which is always good.
Thanks for your help! Also, take your time and replicate this procedure, as it truly is very satisfying, especially with lead, but also with
manganese-assuming there's no relevant iron contamination. As soon as I have time I'll be making a whole series of transition metal formates, and
maybe some double formates as well, but that involves using barium salts, which I never feel like "wasting". Thanks again!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by valeg96  |
No, thanks to you, Nathan. The questions and doubts you raised incited me to do some research, and learn some additional facts, which is always good.
Thanks for your help! Also, take your time and replicate this procedure, as it truly is very satisfying, especially with lead, but also with
manganese-assuming there's no relevant iron contamination. As soon as I have time I'll be making a whole series of transition metal formates, and
maybe some double formates as well, but that involves using barium salts, which I never feel like "wasting". Thanks again! |
Haha, certainly - and that's great! Please do pursue transition-metal-formate salts - besides the fact that I'm at the moment attempting something
similar with acetates, I love getting to see the results. And although it may not be practical, the characterizing of little-known compounds is
definitely my favorite part of home chemistry. Good luck!
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I had reason to prepare a small quantity of manganese II formate and the results I got today were rather different from the compound desribed above.
My manganese formate is a very pale pinkish lilac colour by contrast with the material described above. My compound would appear, therefore, to be a
fairly normal type Mn2+ compound.
I didn't comment on the dark colour of Valeg96's compound before because I know that formaldehyde oxime forms a reddish brown complex with Mn2+ in the
presence of oxygen so I thought that perhaps formate does the same. In reality, I suspect that the brown complex is actually a complex of Mn3+ or
higher valency, stabilized by the ligand. It appears that the Mn2+ formate is quite stables and pale coloured. However, given Valeg96's method,
dissolving MnO2 in formic acid without an addition reducing agent, it is possible that he has prepared the Mn3+ formate. I will try his method
tomorrow.
My procedure was as follows:
20.05g of dark brown, higher Mn oxide that was recoved from a permanganate oxidation was stirred into a slurry with 25ml of water and then 17.5ml of
85% formic acid and 150ml 0f 6% hydrogen peroxide were added slowly and alternately over a period of 10 minutes. 1ml of formic acid was added followed
by 5ml of 6% hydrogen peroxide and then the cycle repeated until all of the acid had been added. After this the remaining hydrogen peroxide was added
as fast as the foaming would allow. The reaction is only slight exothermic and the final solution is barely warm to the touch.
The result was a nearly clear solution with a little suspended black and white solids. The solution was filtered to give a clear, very pale pink
solution. This was placed in a shallow basin on a water bath and evaporated until crystal started to form within the solution in some quantity (about
75ml) and then it was allowed to cool to room temperature (about 12C) when abundant crystals formed. The crystals were disaggregated, filtered at the
pump and dried on a watch glass. I will report the yield shortly and hopefully the hydrate number.

Pale pinkish lilac Manganese II formate
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
OK I have dried and weighed the product from the manganese II preparation and I obtained 20.69g and by reducing the filtrate to about 25ml I recovered
another 2.28g of less pure material. Nothing further could be recovered from the rather viscous residue. The yield assuming a dihydrate is rather
smaller that expect 41.7g from theoretical MnO2 however it is likely that the starting material contains much water as it was only air dried.
I then repeated the preparation with 20.03g of Mn oxide and 40ml of 85% formic acid but without the hydrogen peroxide. The reaction was much slower
and required heating almost to boiling and the addition of much water but eventually I ended up with about 150ml of pale pink solution again. I
filtered this and cooled it directly as it appeared almost saturated anyway and obtained 14.408g of crystals identical to those obtained in the
previous experiment.
From this my conclusion is that there is only one manganese format under normal aqueous condition and that it is very pale pink when pure. So I
suspect Valeg96's brown or apricot coloured crystals are not pure.
|
|
|
|