symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
High Test Hydrogen Peroxide (catalytic)
Step 1
Concentration of 3% to 30 %
https://m.youtube.com/watch?v=5dPTtOimdzk
15%hydrogen peroxide
The clear outer liquid in a glow stick
30% hydrogen peroxide
Pharmacy
Hydroponics
Hair salon(beauty supply)
Step 2
Vaccum distillisation

The water goes into the one way valve at the end of the tube.
Hydrogen Peroxide is left behind
http://www.sciencemadness.org/talk/viewthread.php?tid=64146
Google search for anhydrous Hydrogen peroxide
Or High test Peroxide has interesting patents
Method 1
Extraction with p-cymene
http://www.prepchem.com/synthesis-of-hydrogen-peroxide/
80-90% hydrogen peroxide starting material can also be obtained by mixing 30% hydrogen peroxide solution with twice its amount of p-cymene.
____________________________
Method 2
Extraction with serine
https://www.researchgate.net/publication/232377047_Preparati...
The adduct of hydrogen peroxide and serine is unstable in these organic solvents
________________________
Method 3
Extraction with Boric acid alcohol ester
http://www.freepatentsonline.com/2386484.html
https://www.google.com/patents/US2386484
Experimental
As stated from
http://www.sciencemadness.org/talk/viewthread.php?tid=14750
Fairly concentrated H2O2 can be prepared by treating a thick suspension of zinc peroxide with H2S, then filter off the ZnS formed.
Another link
http://www.sciencemadness.org/talk/viewthread.php?tid=1325
Experimental
Urea/peroxide adduct
Add citric acid form
Hydrogen peroxide and urea citrate percipitate.
And urea citrate is supposedly insoluble and not destructive to hydrogen peroxide
Urea peroxide and citric acid is a solid and water is added to leach out the peroxide and drive to precipitation reaction of urea citrate
Not sure if this could work in absolute ethanol without reacting
Ethanol has a lower boiling point the pure hydrogen peroxide.
[Edited on 17-6-2017 by symboom]
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
The extraction with cymene seems quite interesting as apparently cymene can be prepared by boiling together limonene and sulfur, both of which are
readily available. I've attached a paper that may help if someone wants to go down this route.
Attachment: cymene from limonene and sulfur.pdf (1.3MB)
This file has been downloaded 805 times
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
needing alot of peroxide
Making this various inorganic peroxides
Peroxide is mixed with each to form the corresponding peroxide dont need high test peroxide for it but why not.
Lithium peroxide
LiOH
Magnesium peroxide
MgO
Calcium peroxide
CaCl2 +NH3
Copper peroxide
CuSO4 + NH3
Zinc Peroxide
ZnO
Finally a lead
Describing use of organic solvents to make anyhydrous solution so the using ethyl ether for extraction was actually true
First google search
ether and sodium percarbonate concentrated peroxide
Long link sorry
Titled safe use of hydrogen peroxide in the organic lab
PDF
https://www.google.com/url?sa=t&source=web&rct=j&...
Solutions of H2O2 in organic solvents: Hydrogen peroxide has good solubility in many organic
solvents and experimenters unable to access the highly concentrated (e.g., 90% or ~25-30M)
grades of H2O2 described in many procedures for synthesis of peracids or alkyl hydroperoxides,
15 have sometimes turned to "anhydrous" solutions available by extraction of 30 or 50% aq. H2O2
into ether or dichloromethane, followed by drying over Na2SO4 or MgSO4.
These solutions, which can be up to 3M in peroxide, should be considered inherently unstable. Our group has
frequently prepared these solutions, but rarely on volumes greater than 10 mL. Ethereal solutions of H2O2 should never be concentrated or stored, but
immediately consumed or
quenched. A recent article describes safety issues related to generation and ignition of O2 head
gas within organic solutions of H2O2.
Attempts to further concentrate ethereal solutions of H2O2 through evaporation or distillation
represent needless hazard; distillations in particular have been shown to be ineffectual in
generating pure material. A recently described alternative involves dissolution of amino acid
perhydrates into organic solvents.
[Edited on 17-6-2017 by symboom]
[Edited on 17-6-2017 by symboom]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I wonder if there is any possibility of creating undesirable amounts of phosgene if extracting with DCM.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
hydrogen peroxide from glow sticks is desolved in an organic layer it doesnt want to react with anything I even added permnganate to it Its like the
organic compound protects the peroxide from attack also it doesnt free the peroxide easily I added it to bleach added it to copper sulfate still no
reaction
Unless the concentration is very low
It was bubbling but only at the boundary layer
Next up add hydrogen peroxide to it maybe I can absorb the hydrogen peroxide out of a 3% solution and see if I can get urea to desolve in it to form
urea peroxide Also need a solvent to blend the two layers together maybe I will add ethanol or ethylene glycol
I appologize my post are long I want to be covering as much as I can experimently as well as cover the topic completely
This photo is my attempt to use the hydrogen peroxide from the glow stick to make copper peroxide blue solution is tetramine copper peroxide

Quoting nitro-genes
http://www.sciencemadness.org/talk/viewthread.php?tid=971&am...
yield of hydrogen peroxide on basis of percarbonate is not that high, but the precess works, even with 30% percarbonate laundry cleaner. Basically,
you neutralize the percarbonate with 37% sulfuric acid at room temperature in a large container while stirring, until most of the percarbonate has
dissolved, liberating the hydrogen peroxde. Then you filter out the undissolved carbonate/percarbonate and add an equal volume of ethanol or
methylated spirits and put it overnight in the freezer, this will precipitate almost all of the sodium sulfate as a hydrate (the loss of hydrogen
perxoide due to forming an adduct with the sodium sulfate is unknown, but probably existent). It is important that the solution is near neutral (or
better, slightly acidic to stabilize the H2O2), since sodium hydrogen sulfate will refuse to precipitate and the ethanol will just form an unmixed
layer on top of the HSO4 solution. After some mixing and overnight cooling, the massive amount of sodium sulfate hydrate formed is filtered out and
the solution boiled to drive off most of the ethanol. The hydrogen peroxide solution formed can probably be used for most reactions, long term
stability unknown.
 
Adding 3% hydrogen peroxide to the organic layer of the hydrogen peroxide glow stick as you can see the organic layer did not absorb the hydrogen
peroxide solution the peroxide solution from the glow stick went into the hydrogen peroxide layer I think more concentrated hydrogen peroxide formed
there is a bit of turbity in the water layer so I boiled some water in a mug then put the test tube in it it cleared up the turbity tgen I shoock the
test tube again I see bubbles forming the boiling water should not have been used should have used hot water I see a precipitate formed between the
boundary of the organic and water layer not sure what it is. my next try is freezing the mixture did not freeze organic layer thickened
In the picture half of the organic layer is gone I presume that hydrogen peroxide has been leached from the organic layer
>> update organic layer soluble with ethanol (alcohol)
Also when organic layer is added to water the water floats on top of the organic layer they do not mix when only when ethanol is added they mix
together no separation occurs
These solutions seem to react with manganese dioxide slowly
Next test distilled water and organic layer
[Edited on 20-6-2017 by symboom]
Cant seem to pull the hydrogen peroxide out something is desolved in the aqueous phase
Because I got it to desolve in ethanol I figure ill use urea to form urea peroxide this is my last thing ill try with the glow sticks.
Process desolve organic layer in ethanol then add urea to form urea peroxide
[Edited on 20-6-2017 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
:-( failed extracting hydrogen peroxide from glow stick by mixing it with ethanol then adding urea to form urea peroxide
It crystalized out but did not for the urea peroxide
Unfortunately only urea crystals formed
Hopefully someone can succed where I failed on the extraction of hydrogen peroxide from glow stick
 
Whats up next to try
A water in insoluble desicant
activated charcoal
Added rapid bubbling also did that with just water
So not sure if it decomposing
Calcium sulfate
It formed an insouble precipitate cloudy suspension
The calcium sulfate has settled to the bottom
Haven checked if concentration increased yet
Silica beads
molecular sieves
Has anyone tried it
Also instead of evaporation in the open dessicant it
Using something like sodium hydroxide
>Concentrating hydrogen peroxide by borate ester
Boiling off the azotropic ethanol which should remove 5% water
Just as the hydrolysis removes more water.
>Extraction of sodium percarbonate with sulfuric acid
>Concentration >extraction with cymene
[Edited on 20-6-2017 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Interesting videos
Vacuum distillisation
https://m.youtube.com/watch?v=jurYOOHDjx0
Evaportaing
https://m.youtube.com/watch?v=_L2ecuDZwlA
Desicant method
https://m.youtube.com/watch?v=1OiAm65hAoo
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
The peroxide is usualy in the glass vial in the middle, should start there.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Yea i dont know why people think that i used hydrogen peroxide to test both solutions it was the outside vial the dye in the glass vial reacted with
the peroxide demostrating that it contains no peroxide also tested with copper sulfate
Hydrogen peroxide does not like to be alone haha
In water it is hard to pull out of the mixture with water
In organic solvent with glow stick water wont pull it out
A vacuum desiccator seems to be the second best method behind vacuum distilization
[Edited on 30-7-2017 by symboom]
[Edited on 30-7-2017 by symboom]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Cymene is fairly easy to obtain and can be extracted from cumin and thyme. I would definitely give the cymene route another look.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Here is a better vacuum distillation setup using glass tubing
Vacuum produced by a asperator adaptor
https://m.youtube.com/watch?v=tYLlkTDstmo
Room temperature boiling water with asperator if it was hydrogen peroxide the water would boil off.
or a hand pump vacuum see picture above

Hot water could be in the wine glass providing some heat
Hard plastic tubing may work since it is low temperature or room temperature and only water is passed over
When I say better I mean better than my original post there is no receiving flask only a long hose with a one way valve and the water collects in
there
Also thanks for the info However, standard Erlenmeyer flasks are not designed to be used under pressure. A filtering flask without a side arm should
be used - these are able to withstand a vacuum.
Or even using two vacuum flasks
The main objective is to figure out a cheap way to do vacuum distillation
[Edited on 8-3-2018 by symboom]
[Edited on 8-3-2018 by symboom]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Anything with a conical flask in it is not a "better" vacuum setup.
(Except in the unusual circumstances of it being a vacuum rated flask.)
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Self concentrating hydrogen peroxide making device
Concentration success a self sealing bell jar with a shot glass of desicant. It is working No heat was applied it may dedicate faster if heated then
closed. The bonus is the descant can be seen through the glass.
The self sealing action is great because it shows if you have a good seal makes a pop sound as the pressure drops inside due to the descant absorbng
the moisture.
After a good concentration then I'll test on cottonballs
[Edited on 27-10-2018 by symboom]
[Edited on 27-10-2018 by symboom]
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Do you really want to combine those in a sealed jar like that?
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
OK then thanks for changing my mind 
Link to pdf
n Thursday May 28, 2009, at approximately 7:30 p.m., a 56-year-old chemist received
chemical burns over 65% of his body when he mixed sodium hydroxide with hydrogen
peroxide.
It does work unfortunately very well  but the decomposition of the hydrogen
peroxide does worry me now so vacuum desication I do not like due to the dangers associated with it. but the decomposition of the hydrogen
peroxide does worry me now so vacuum desication I do not like due to the dangers associated with it.
Calcium chloride descant is more suited for this
[Edited on 28-10-2018 by symboom]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A 'cool' way to concentrate H2O2, to quote:
"The freezing points of H2O2 and H2O are very similar, so this is tedious, but it is quite effective. Put the solution in the freezer and skim off ice
crystals as soon as they form. The ice crystals, if small, will be nearly pure water. This will increase the H2O2 concentration of the unfrozen
portion."
Source: https://www.quora.com/What-is-the-easiest-to-way-purify-H2O2...
--------------------------------------------------
Perhaps of only theoretical interest, very 'High Test Hydrogen Peroxide' can be formed, at least in small amounts, to quote a source (see 'The gas
phase reaction of singlet dioxygen with water: A water-catalyzed mechanism' by Xin Xu, Richard P. Muller, and William A. Goddard III, in PNAS March
19, 2002 99 (6) 3376-3381; https://doi.org/10.1073/pnas.052710099:, link: http://www.pnas.org/content/99/6/3376):
"Recently, Wentworth et al. (1, 2) from the Scripps Research Institute reported the surprising result that Abs, regardless of source or antigenic
specificity, have an ability to catalyze the generation of H2O2 in a highly efficient manner and by a mechanism that involves the oxidation of H2O by
singlet oxygen molecules, O2 (1Δg). ..... To understand how Abs can carry out this remarkable and unexpected chemistry, we need to understand how 1O2
can interact with H2O to produce H2O2. Preliminary mechanistic investigation (2)§ suggested that 1O2 might be able to convert H2O to H2O2 via the
formation of hydrogen polyoxides, H2O3 and H2O4."
where one might call H2O3 and H2O4 high test grades of hydrogen polyoxides.
Note: working with small quantities is a very good idea when working with potentially energetic materials.
[Edited on 30-10-2018 by AJKOER]
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
ABS as in ABS plastic?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
No, apparently the reference to Abs (or singular Ab), upon research I believe refers to either sticky protein structures, see to quote https://www.scripps.edu/news/old/press/021308.html:
"the proteins that stick together are amyloid-beta (Ab), proteins that do not affect the heart. TTR protein, which is made in the liver to transport
vitamin A and the thyroid hormone through the bloodstream, has rarely been sighted in brain tissue. But there have been scattered research studies
over the past 15 years that have found TTR can bind with Ab, thereby preventing Ab molecules from clumping together."
or possibly more generally, micro structures, like discussed in this article associated with a virus platform: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4039020/ .
I will leave the final word to any resident microbiologist.
[EDIT] See also comments at https://www.ncbi.nlm.nih.gov/pubmed/26855676 . To quote:
"A pathway for the formation of both O3 and hydrogen peroxide (H2O2) was previously proposed, beginning with the antibody or amino acid-catalyzed
oxidation of water by singlet oxygen ((1)O2) to form hydrogen trioxide (H2O3) as a key intermediate. A key pillar of this hypothesis is that some of
the H2O2 molecules incorporate water-derived oxygen atoms. However, H2O3 decomposes extremely readily in water to form (1)O2 and water, rather than O3
and H2O2. This article highlights key literature indicating that the oxidation of organic molecules such as the amino acids methionine, tryptophan,
histidine, and cysteine by (1)O2 is involved in ozone formation. Based on this, an alternative hypothesis for ozone formation is developed involving a
further reaction of singlet oxygen with various oxidized organic intermediates. H2O2 having water-derived oxygen atoms is subsequently formed during
ozone decomposition in water by known reactions."
See also full text available at https://core.ac.uk/download/pdf/33115718.pdf which discusses the action of (1)O2 on water as proceeding either by one of two pathways:
(1)O2 + 2 H2O = 2 H2O2
Or:
2 (1)O2 + 2 H2O = 2 H2O2 + (3)O2
[Edited on 2-11-2018 by AJKOER]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Using
Serine
Hydrogen peroxide is one of the most versatile oxidation reagents, still it has not fully been exploited by synthetic chemists since anhydrous (let
alone pure) hydrogen peroxide requires hazardous preparation protocols. We have recently reported on the crystallization of serine and other amino
acid perhydrates, thus paving the way for a new method for laboratory-scale production of anhydrous hydrogen peroxide solutions. Serine is insoluble
in most organic solvents (e.g., methanol, ethyl acetate, and methyl acetate) that readily dissolve hydrogen peroxide. Moreover, since the adduct of
hydrogen peroxide and serine is unstable in these organic solvents, crystalline serine perhydrate readily decomposes to give anhydrous solutions of
hydrogen peroxide and crystalline precipitate of the amino acid. This procedure can then yield an anhydrous hydrogen peroxide solution in a single
step. Moreover, filtration of the amino acid, and room temperature evaporation of the volatile solvent (e.g., methyl acetate), yields over 99%
hydrogen peroxide.
Using Cymene
another method 80-90% hydrogen peroxide starting material can also be obtained by mixing 30% hydrogen peroxide solution with twice its amount of
p-cymene, followed by distillation of the mixture at 50°C, using an aspirator vacuum. Most of the water and p-cymene is thus removed. After
mechanical separation of the remaining p-cymene-H2O2 mixture.
Using Boron alcohol ester
Anhydrous solutions of hydrogen peroxide in alcohols are stable at room temperatures, they are not explosive but burn with a smoky flame.
Anhydrous solutions of hydrogen peroxide in an alcohol may be prepared by the treatment of a boron ester of the alcohol with an aqueous solution of
hydrogen peroxide. In general, that 4 amount of hydrogen peroxide is employed to react with the ester of boric acid which contains sufficient water to
hydrolyze the ester to boric acid. The reaction takes place between the ester and the peroxide solution in either alkaline neutral or acid solutions
and generally at moderate to low temperatures.
react upon the desired boron ester with the precipitation of relatively insoluble boric acid and the formation of the alcohol containing dissolved
therein the hydrogen peroxide initially present in the reacting aqueous solution. The reaction takes place in accordance with the following equation:
B(RO)a + --- + B(OH) Swhere 1R is an alkyl radical and n is the molar concentration of hydrogen peroxide in the quantity of aqueous hydrogen peroxide
employed. It will be noted from the equation that simple hydrolysis of the ester occurs. In the absence of water, there is no reaction between
hydrogen peroxide and the ester.
The amount of hydrogen peroxide in the alcohol produced by hydrolysis of the ester is dependent on the concentration of the hydrogen peroxide in the
original aqueous peroxide solution and the molecular weight of the ester. Reacting esters of lower molecular weight will produce a higher
concentration of peroxide in the alcohol.
If the water in the aqueous peroxide solution be in excess of the stoichiometric amount, two layers are obtained in the reaction mixture after the
boric acid is filtered off. There is thus formed an upper layer consisting of a solution of hydrogen peroxide in the alcohol and a lower layer
consisting of a solution of hydrogen peroxide in water. The amounts of hydrogen peroxide in the two layers depends upon the distribution coefficient
of hydrogen peroxide between the alcohol and water.
If The amount of water in the hydrogen peroxide solution is less than the stoichiometric amount, the solution will comprise of a single layer
consisting of the alcohol having hydrogen peroxide and the excess boron ester dissolved therein. In the case of esters of the low molecular alcohol,
such as methyl and ethyl alcohols, only one layer is obtained as these alcohols are miscible in all proportions with water.
Example 1 38.4 grams of normal butyl borate were reacted with 31 grams of 57% hydrogen peroxide solution and permitted to stand overnight as in
Example 1. After filtering off boric acid, 51 grams of solution were obtained of anhydrous hydrogen peroxide in normal butyl alcohol. This solution
contained 14.1% active oxygen which represented a 30% hydrogen peroxide solution.
Example 2 The boron ester of benzyl alcohol is not reported in the literature. It is, however, possible to prepare this compound by reacting benzyl
alcohol with boron anhydride at water bath temperature under reduced pressure.
Example 3 60 g. of benzyl borate were treated with 20 ml. of 100 vol. (27% by weight) hydrogen peroxide.
30 3. The process of preparing a solution of hydrogen peroxide in amyl alcohol which comprises reacting amyl borate with sufficient aqueous hydrogen
peroxide to hydrolyze the borate to boric acid.
65 4. The process of preparing a solution of hydrogen peroxide in butyl alcohol which comprises reacting butyl borate with sufficient aqueous hydrogen
peroxide to hydrolyze the borate to boric acid.
60 5. The process of preparing a solution of hydrogen peroxide in isoamyl alcohol which comprises reacting isoamyl borate with sufficient aqueous
hydrogen peroxide to hydrolyze the borate to boric acid.
Interesting videos
Vacuum distillisation
https://m.youtube.com/watch?v=jurYOOHDjx0
Evaportaing
https://m.youtube.com/watch?v=_L2ecuDZwlA
Desicant method
https://m.youtube.com/watch?v=1OiAm65hAoo
[Edited on 25-10-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Here are some other reactions
For hydrogen peroxide synthysis
http://oxygen.atomistry.com/hydrogen_peroxide_synthesis.html
Here is a video of hydrogen peroxide from sodium peroxide
https://youtu.be/LB2pO7OteDU
Hydrogen peroxide from barium peroxide
https://youtu.be/THuBQV96RKM
I've been trying to find reactions that don't require
hydrogen peroxide being separated by distillation under reduced pressure.
This is how freezing out to concentrate the solution works
Hydrogen peroxide and water form a eutectic mixture, exhibiting freezing-point depression down as low as –56 °C; pure water has a freezing point of
0 °C and pure hydrogen peroxide of −0.43 °C. The boiling point of the same mixtures is also depressed in relation with the mean of both boiling
points (125.1 °C). It occurs at 114 °C. This boiling point is 14 °C greater than that of pure water and 36.2 °C less than that of pure hydrogen
peroxide.
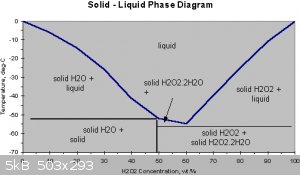
Credit goes to the picture made by another user
Potassium hydrogen tartrate and potassium fluosilicate
are sparingly soluble substances, so that concentrated aqueous solutions of hydrogen peroxide may be prepared by treating potassium peroxide with
concentrated solutions of tartaric acid or hydrofluosilicic acid. Barium peroxide, however, is the substance commonly employed.
Sodium percarbonate can react with sulfuric acid to form sodium sulfate which can be separated out by chilling the solution and Hydrogen Peroxide
Or hydrogen peroxide can be reached out with a solvent.
Potassium peroxide is treated with a solution of tartric acid
Which forms insoluble potassium tartrate and hydrogen peroxide of a concentration depending on how much water is used for the reaction
I guess I'll bite the cost to satisfy my curiosity
Materials
Methyl acetate
L-serine
35% Hydrogen peroxide
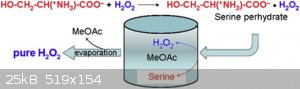
[Edited on 7-11-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Here is synthysis of barium peroxide from prepchem.com
1) Barium peroxide
Twenty-six grams of barium nitrate are placed in a large covered crucible and heated with slowly rising temperature to a dull red color (about
500°C) for about one-half hour. After cooling, the mass is broken up quickly into small lumps and transferred rapidly to a weighed combustion tube.
The tube and contents are weighed to determine the amount of barium oxide present and then heated in a circular combustion oven at about 460°G in a
steady stream (about 3 bubbles/sec) of air which has been dried by passing first through sodium hydroxide and then through concentrated sulfuric acid.
After cooling in the air current, the tube is weighed; the increase in weight should correspond to one-tenth the initial weight of the barium oxide,
otherwise the material must be reheated again in oxygen using a slightly higher temperature (500° C).
2) Sodium peroxide
Li +NaCl -> LiCl + Na
Na + O2 --> sodium peroxide
I'd probably make sodium metal from lithium metal and sodium chloride. Distill the sodium metal then oxidize the sodium metal in pure oxygen
Sodium peroxide
I thought of an experiment to sodium percarbonate
From sodium peroxide and sodium bicarbonate which should lower the pH enough to prevent decomposition and form sodium carbonate in situ upon which the
sodium carbonate adsorbs the hydrogen peroxide forming an adduct.
3) Percarbonate/Perborate
I plan to do extraction of hydrogen peroxide from percarbonate and perborate using ethyl acetate which seems due to the research able to dissolve
hydrogen peroxide from it's adduct.
4.1) Urea peroxide
Reacting urea peroxide with calcium or barium hydroxides to precipitate peroxide, leaving urea dissolved, and, after filtering out the alkaline earth
metal peroxide, recovering hydrogen peroxide from it with sulfuric acid.
(4.2)
I'm not sure if urea is soluble in ethyl acetate
But this could be an interesting path to Hydrogen peroxide.
5) Zinc peroxide
The electrolytic preparation, A solution of zinc chloride, neutralised by adding zinc oxide, is electrolysed in a vessel containing a porous
diaphragm, and hydrogen peroxide is added to the cathode cell. The precipitated hydrated peroxides are carefully dried.
Or a similar preparation to calcium peroxide
Fairly concentrated H2O2 can be prepared by treating a thick suspension of zinc peroxide with H2S, then filter off the ZnS
6)Calcium/Strontium peroxide
Five grams of anhydrous strontium nitrate (or the equivalent weight of the 4-hydrate) are dissolved in 50 ml of 3% hydrogen peroxide and mixed with a
solution of 7 ml of concentrated aqueous ammonia in 100 ml of water. As described under the procedure for barium peroxide the SrO2 8-hydrate is
converted to the anhydrous compound by heating it at 800°C for about 4 hours in dry carbon dioxide free oxygen. The yield of SrO2 is
2.5-3 g.
Calcium chloride could be substituted to form calcium peroxide even though contact with water hydrolyzes with release of oxygen. Treatment with acid,
it forms hydrogen peroxide.
_________
Another interesting one is Sodium peroxynitrate which can be made from an alkaline solution of sodium nitrate and hydrogen peroxide. The solution is
evaporated until crystals start to form, and the solution is then mixed with alcohol, causing crystals of the octahydrate to form
I've done some tests with 35% hydrogen peroxide on aerogel and activated carbon
With activated carbon it decomposed
With silica aerogel it doesn't absorb into the structure which suprised me it seems like aerogel is hydrophobic.
---------
Update finally got the serine - hydrogen peroxide adduct
I got some nice crystals. I added ethyl acetate and extracted the peroxide nicely I then added this disolved Hydrogen Peroxide in ethyl acetate then
added it to a cotton ball and lighted it on fire it burned like nitrocellulose.
Google search
Site:prepchem.com peroxide has lots of hydrogen peroxide adducts
Hydrogen peroxide is so useful able to improve yield of nitric acid and assisting in oxidizing sulfur dioxide to sulfuric acid.its use as piranha
solution and its ability to assist in desolving metals when combined with an acid like nickel and copper.
Serine peroxide adduct
 
[Edited on 22-11-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
I'm still curious on hydrolysis of ammonium persulfate in a pressurized container such as a pressure cooker if this can produce hydrogen peroxide that
would be another path.
Can't find any information on it.
Since my other links are dead
(Hate how much information on chemistry is censored, hard to find or behind paywalls)
On addition of 30 per cent, peroxide to sodium hydroxide or ethoxide a precipitate of sodium perhydroxide is obtained to which the formula 2NaHO2.H2O2
is given. This possesses marked basic properties, and on saturation with carbon dioxide yields the acid percarbonate, NaHCO4. The same compound is
formed when hydrogen peroxide is added to sodyl hydroxide, NaO.OH. This latter substance was first prepared by Tafel by the action of sodium peroxide
upon well-cooled absolute alcohol.
With potassium hydroxide two compounds have been obtained, namely, 2KHO2.H2O2 and 2KHO2.3H2O2.
On passing dry ammonia into a solution of pure hydrogen peroxide in absolute ether at -10° C., or into pure anhydrous peroxide at 0° C., ammonium
perhydroxide, NH4O.OH, or NH3.H2O2, crystallises out. Further addition of ammonia is stated to convert this into an oily mass which solidifies at
about -40° C. and has the composition (NH4)2O2, and is therefore ammonium peroxide, the analogue of sodium peroxide. The crystals of ammonium
perhydroxide melt at 24.5° C. to an oily liquid, which is stable in the entire absence of water.
Reactive chem
https://www.bitchute.com/video/NbX4SWk9a1hA/
Concentrating 3% hydrogen peroxide by
Dessication with calcium chloride
Distillation under reduced pressure with sulfuric acid
Synthesis of hydrogen peroxide
https://youtu.be/LB2pO7OteDU
Sodium peroxide
https://youtu.be/ZqEWUw6sgpA
Potassium peroxide
Several peroxides have been described. The tetroxide, K2O4, or K2O2,O2, is produced by burning the metal in air or oxygen, de Forcrand heats the
potassium first in a current of nitrogen, then in air, and finally in pure oxygen at a temperature between 180° and 200° C. The product is a
sulphur-yellow, very hygroscopic powder, its heat of formation from the elements being 137.74 Cal. It is decomposed by water, with evolution of oxygen
and formation of potassium hydroxide and hydrogen peroxide:
Urea peroxide
https://youtu.be/qoqMHbxN4i8
one method involves using calcium or barium hydroxides to precipitate peroxide, leaving urea dissolved, and, after filtering out the alkaline earth
metal peroxide, recovering hydrogen peroxide from it with sulfuric acid. This, however, requires a very concentrated solution of the adduct, decanted
or vacuum-filtered from a sludge of wet powdered percarbamide.
Sodium percarbonate
https://youtu.be/yHATAwpfSTU
Sodium borate added to glacial acetic acid will yield peracetic acid, which can be used to oxidize aromatic amines to nitro groups,
Sodium perborate & barium peroxide
https://youtu.be/THuBQV96RKM
Sodium perborate 1
https://youtu.be/DtKRwcweVow
Sodium perborate2
https://youtu.be/cJ2DxN5Ly0Y
Properties of high test Hydrogen peroxide
https://youtu.be/hJSWTPOa4T8
Concentrating and measuring
https://youtu.be/nVAe__ToAOY
http://antenna-theory.com/high-test-peroxide.php
I hope to write a comprehensive guide on Hydrogen peroxide after a few experiments.
[Edited on 25-11-2020 by symboom]
[Edited on 25-11-2020 by symboom]
[Edited on 25-11-2020 by symboom]
|
|
|