Zool
Harmless

Posts: 18
Registered: 6-1-2017
Member Is Offline
Mood: No Mood
|
|
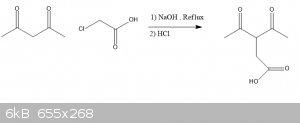
Acetyl acetone as nucleophile
I am trying to do this reaction
any suggestions ?
Will it work?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4363
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
You'd be better off using ethyl chloroacetate than chloroacetic acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zool
Harmless

Posts: 18
Registered: 6-1-2017
Member Is Offline
Mood: No Mood
|
|
but if I use ethyl chloroacetate dont I risk a claysen condensation as a sidereaction ?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4363
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No, because the Hacac is far more acidic than the ester.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
A bit more information would be nice. Is it your idea or somewhere from the literature? What solvent do you have in mind?
First, don't overdo it with the base and reflux. Acetyl acetone already has a fairly high equilibrium ratio of enol, but the ratio depends on the
solvent. In water there is even more enol than ketone. So if you feel brave, try the reaction with just as much base as needed to neutralize HCl,
e.g. NaOH, Na2CO3.
https://en.wikipedia.org/wiki/Acetylacetone#Properties
Therefor I would try the following ratio:
1 eq. acetyl acetone + 1.1 eq chloroacetic acid + 0.55 eq. anhydrous Na2CO3 (anhydrous because this way you get the exact amount you want) or 1.1 eq.
NaOH
This means, the base in your reaction mixture will be sodium chloroacetate! Prepare the salt in solution, then add acetyl acetone and stir. If you
plan using ethanol as the solvent do not run the reaction completely anhydrous as it's known for sodium acetate that some equivalents of water enhance
solubility effectively. I think this is true for chloroacetate as well.
Start with a trial at 40 °C or so and concentrated solution. If that's not enough go up to 50 °C and so on. I'd check if water is a suitable
solvent, ethanol or a mixture.
No guarantee this will work and of course this doesn't mean it won't work otherwise.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
You will get 4,6-DIOXOHEPTANOIC ACID
Synthesis of dioxocarboxylic acids
Pendarvis,R.O.; Hampton,K.G.
Journal of Organic Chemistry, 1974 , vol. 39, p. 2289 - 2291
DOI:10.1021/jo00929a035
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
@Waffles. Your literature source displays a special case, which is achieved by generating disodioacetylacetone.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
You should use twice excess of NaOH, to transform both acetyl-acetone and chloroacetic acid in their Na-salts. Then they will react by the scheme:
(CH3CO)2CHNa + ClCH2COONa = (CH3CO)2CH-CH2COONa + NaCl.
And at the last step the product (Na-salt) should be neutralized by 1 equiv. of an acid (HCl, AcOH etc.) to obtain your product.
|
|
|