| Pages:
1
2 |
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
My copper tetra Amine Adventure
The best of run off chem and eye balling!
When cruising you tube I came across some testing of the above substance and figured it was nifty and cursory look into its making was mentally
shelved for later use.
https://www.youtube.com/watch?v=YaCLTWsHEpI < Setting off
https://www.youtube.com/watch?v=Uj9BkyGo70w < One method of synthesis
https://www.youtube.com/watch?v=X65pAuLGlDo < Synth 2 (Basic method I am using)
Well Using the Nile reds method of making zeotropic Nitric acid such as adding water to the nitrate salt then reacting with the H2SO4 then re
distilling collecting at 120c.
Nile red method: https://www.youtube.com/watch?v=KBeo8nww21g
Well he kept the weak fraction coming under the 120C mark for cleaning, but I decided to use scrap copper and make copper nitrate, Ok nifty but what
to do with it?
Well I happened to have Ammonia on hand, at 3% So dump the two in and well pretty no crystals too much water.
Back to the nitric acid: From wally world you can get CaN/AM cold packs, So to get rid of the Ca I react with the Ammonia, I keep adding small amounts
till no more precipitate forms leaving me with aqueous AN SO via Nile red method I just skip the water and pour in this now pure AN solution! Takes 2
boxes (4) cold packs to make 250Ml 68% nitric (Put the CaOH tot he side it can be handy)
So we have (NH4)2SO4, HNO, H2O, So set aside the HNO/H2O and set off to dehydrate the (NH4)2SO4 to a concentrated solution, so I set that aside and
store for what ever.
Back to the tetra amine: So now I have my some what deep blue solution and some greenish precipitate, So too much water and not enough ammonia so what
to do? Ah ha! yes I have Sodium hydroxide. So lets make a NH3 generator.
Dramatic Re-Enactment:
 
As you can see it was a good success but needed more ammonia but ran out of Sodium hydroxide, so back in the fridge it goes till I can get more. You
can see the ammonium Sulfate has some minor contaminant but this has no effect on the gass generation.
The check valve is just a cheap air check valve to prevent suck back or rather slow it down (Remember you get what you pay for!)
But that is my interesting story of how to use waste products from one reaction to fuel another!
Will update as the adventure continuous! Looking forward to the end testing! Need a nice big pumpkin to soften up for some food grad chemistry!
[Edited on 2-4-2017 by XeonTheMGPony]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
if you can obtain, increase concentration NH3 from 3 on 24%, you can use method "one container" . Process takes 1 hour.
https://www.youtube.com/watch?v=pSbU1DDKivg
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
That's the point of the ammonia generator, I simply bubble pure ammonia gas into the all ready present solution of 3% I used at the start with no
additional liquid.
Basically skipping the middle man, this is mostly a "use up waste products" from my main reaction of making concentrated Nitric acid and its
azeotrope.
Very hard to find concentrated ammonia in these parts.
But interesting work up when it is the target substance!
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 193
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
So are you trying to make tetraamine copper nitrate by the method of mixing ammonia and copper nitrate solution? If you want to use a known method,
then you could just use your ammonia generator to make 24-30% solution and mix that with your copper nitrate, but your method seems fine. Also, if you
have easy access to ammonium nitrate, there is a method using ammonia, CuO and ammonium nitrate. Anyway, good experiment and I look forward to seeing
this finished. (I may repeat this next time I get the opportunity to make nitric acid, I am currently out and very busy)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Like I said I was aware of the methods all ready there, but I wanted to find a use full way to dispose of waste products of my main reaction which was
the Nitric acid.
So when you look at the over all reactions, you can skip some of the middle men  So I take my header from the azeotropic distillate toss in scrap copper and let that react a few days til no more copper reacts.
So I take my header from the azeotropic distillate toss in scrap copper and let that react a few days til no more copper reacts.
Take out the copper, and chill in the fridge.
Take my fresh ammonium sulphate to make the NH3, I take my flask dump in NaOH till 1/4 of the bottom is full then drip in my (NH4)2SO4 till it stops
producing gas, then reset and run again till sufficient ammonia is in solution.
Seems to work quite well, I just didn't have as much Hydroxide as I thought I did.
You definitely need to chill the copper nitrate solution befor ammonia addition and stirring helps but the bubbles will do a bit as well as steam is
generated.
I just wanted to show a method to Jerry rig up an addition system when one lacks an addition funnel and such for a gas generator.
Thanks for your interest  Will try to get pics of the generator running next
time. Will try to get pics of the generator running next
time.
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 193
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
How hard to detonate is TACN anyway? I know it will deflagarate with strong heat but how would one detonate it?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
300mg HMTD or SADS for any weight of main charge.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Got more NOH so this is how it looks after the second absorption run, I can now smell strong free ammonia from the solution, will do a third run once
cool to saturate it.
All the material is now a nice dark blue with just a few specs of the lighter blue precipitate.

|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
It seems, that your big problem is obtained ammonia water 24%. It is usual concentration for store and for next use. Instead it for you is not problem
obtained HNO3. Notable country. Well, last way anyway, concentration ammonium water on 25% or more, is necessary at zero celsia adding NH3 gas.
After increase NH3, will precipitate crystals TACN directly from solution. At zero C and concentration 28% will in jar pretty light blue water and
huge amount TACN on bottom. Better is cooling solution for example on - 18C. Results will still better. And solubility NH3 of course highest.... ...Dr. ...Dr.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
unless your red seal trades or a science department you can't get any thing of use able value here it seems.
Thank fully I just make my own! So hence the direct absorption of ammonia gas (I dislike middle men!)
By cool I mean fridge and or freezer, or I will state to room temp as room temp is rarely ever cool! (For me 16/17c is hot and preferred temp, every
one seems to like it on the sun at 21!!!!)
Never got the third run finished, got distracted with making more 68% nitric, and getting the petroleum ether dried and re distilled in prep for the
hexamine cleaning and HDN synth. The horror I have let my H2SO4 stocks get low and must buy and distill more drain cleaner 17 dollars a L here! food
first so may be a while.
So far I have noted the best efficiency for the gas generator has been to dissolve the NOH in a bit of water along with the Ammonium sulfate, Makes
for a smoother and fuller reaction then heat once it is don reacting on its own
The check valve has been doing an amazing job at stopping any suck back, I'll benefit greatly by having a buffer on it though will have to see how
well a generic rubber balloon will hold up! just needs to survive a single use.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2894
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
What is NOH?
Nickel oxyde hydride? A typo for NH4OH or NaOH?
When using accronyms or abreviations..to avoid any confusion you should write somewhere into your text the name/meaning in extension.
The use of NH3 or NH4OH to get rid of Ca(2+) into Ca/NH4 nitrate is not the best method since Ca(2+) tends to be complexated by NH3...
1°) Remember that EDTA (Ethylen diamine tetraacetic acid) complexates Ca(2+).
2°) Soluble Ca(NH3)x(NO3)2 may form with x = 1,2,4,8... (see complexation of NH3 by Mg, Ba, Ca, ... salts)
So maybe you got some Ca(OH)2 precipitation (what is not that unsoluble BTW) but most of the Ca(2+) did remain into your initial reaction batch. Would
be smarter to use Na2CO3 or NaHCO3 until no more unsoluble CaCO3 precipitates.
Na(+) will not interfere into the Cu(NH3)4(NO3)2 process.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | What is NOH?
Nickel oxyde hydride? A typo for NH4OH or NaOH?
When using accronyms or abreviations..to avoid any confusion you should write somewhere into your text the name/meaning in extension.
The use of NH3 or NH4OH to get rid of Ca(2+) into Ca/NH4 nitrate is not the best method since Ca(2+) tends to be complexated by NH3...
1°) Remember that EDTA (Ethylen diamine tetraacetic acid) complexates Ca(2+).
2°) Soluble Ca(NH3)x(NO3)2 may form with x = 1,2,4,8... (see complexation of NH3 by Mg, Ba, Ca, ... salts)
So maybe you got some Ca(OH)2 precipitation (what is not that unsoluble BTW) but most of the Ca(2+) did remain into your initial reaction batch. Would
be smarter to use Na2CO3 or NaHCO3 until no more unsoluble CaCO3 precipitates.
Na(+) will not interfere into the Cu(NH3)4(NO3)2 process. |
Typo! NaOH! sorry!
I make Nitric acid with the Ammonium nitrate, So if some calcium carries along doesn't matter in the least, but judging from the follow up in the gas
generator and such the amount of Ca that comes along is meaningless as I haven't found any visible evidence of it.
the Ammonia displacement of Ca in the mixed nitrate is don as a saturate environment, and on average every thing comes out near identical to
theoretical for balances, so much so any deviation can be scale error (I didn't spend a grand on a lab scale so that can be a fair margin)
Add some heat to encourage every thing to react and do its thing then vacuum filter the hot saturated solution and left with a Ammonium nitrate w/
free ammonia solution. Further heat to reduce water concentration and off gas as much ammonia as plausible left with clear concentrated Ammonia
Nitrate solution.
Now one can further dry to crystallize, or make azeotropic nitric acid. I've don both.
But I will try the carbonate method, then do a side comparison to my normal method and see what differences are observed, will be an entertaining week
end project!
But will the Na interfere with nitric acid! as the copper tetra amine is a time wasting project long term I see this as rather worthless in practical
blasting, Nitric is my main target, and the Ammonium sulfate by product is use full in other areas so it is the preferred waste stream.
My goals is to focus on industry standard E.M.'s
[Edited on 18-4-2017 by XeonTheMGPony]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Today was filter day, some nice high density crystals followed by some nice fluffy ones!
So Now I need to boil down the mother liquor and hit it with ammonia gas again and add some more copper nitrate to get more crystals till all my waste
copper nitrate is used up from the mercury cleaning!

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2894
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Looks like you finely got TACuN 
To speed up precipitation, collection and drying one may use methanol/ethanol dropped into the Cu(NO3)2 saturated solution and then introduce the
concentrated NH3 solution (NH4OH)...
To speed up things further add a little diethyl-ether...
The cristals may be conserved dry for ages into an hermetical recipient under ether...
The ether layer is clear as water and the cristals very deep blue at the bottom...cristals dry faster when filtrated since ether is very volatile
==> at ambiant heat
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
TACuN
And after all what described Philou, hitting on the anvil. TACuN requires a bigger hammer. For confirming his properties.... ...Dr. ...Dr.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Just weighed out to 38.x grams, will ensure to do so for testing, thanks.
And hey 4 months is nothing when ignoring a beaker lol those crystals been sitting there for ages just haven't bothered to filter them out but with my
shiny new glass powder addition funnel and bag of cotton balls handy figured take it for a test drive
PZ: Good news on the shelf life, I was suspecting they'd be short lived once dried! Hence why I left them in solution till I was ready to play with
them. guess semi-hermetic will have to suffice as I tend not to weld things like ether cans 
LL: Does it need to be wrapped in any thing like tin foil or paper? Or just a good sized crystal and a firm hit?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
TACuN
TACuN if is dry and for example washed in ethanol, not requires any closure in foil or paper. Simply hit by hammer 0,03g crystalls on anvil. Should by
works. For better conditions is possible covered in alu foil. Aluminium powder 3 - 10% increase sensitivity TACuN almost on 2x. Dr.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Would methanol be compatible? As I have scant anhydrous ethanol and would rather not use it on that!
Goat I need to get my vacuum desiccation chamber built! too much to do for one man!
[Edited on 29-7-2017 by XeonTheMGPony]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2894
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by XeonTheMGPony  | Would methanol be compatible? As I have scant anhydrous ethanol and would rather not use it on that!
Goat I need to get my vacuum desiccation chamber built! too much to do for one man!
[Edited on 29-7-2017 by XeonTheMGPony] |
Yes methanol compatible although a little more soluble inthere than into ethanol....maybe isopropanol could be another possibility but I have no
experience with it...theorically should be better or equal to ethanol ;-).
Avoid aceton since it reacts with ammonia and will change the properties of the complex.
Spare the good ethanol to celebrate succes 
Better use hermetic jar because otherwise the vapour pressure of TACuN will set NH3 free...any conserving food jar like jam jar, bolognese salsa jar,
... will do...after washing with liquid soap, warm water, rinsing and drying 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Well I pressed into a card board tube as hard as the tube would with stand, used a 1 gram etn booster and made a nice hole in the dirt!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1457
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
TACuN
OK, good attempt. How I say, TACuN requires pretty hitting. ETN 1g is maybe too, but for confirmed full strength also enough 0,2 ETN should by give a
full VoD, which is about 3,5 Km/s. In any cavity. Dr.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1049
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Why TACN?? the perchlorate is so much cooler.
Can someone please try to grow a large crystal of the TACP, it is so pretty, plus I would like to measure the det properties of a monocrystalline
explosive. And they are so pretty. Any tips on how to do this other than cooling the solution slowly 
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
The results!
  
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2894
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nice but to be more speaking and representative it is good to have a reference object next to the hole and the initial charge too.
So one can have an idea of the size of the hole vs charge vs reference  
==> Nothing look more like a hole than another hole without references (2cm, 10 cm, 50cm, 1m...hard to tell)
Laboratory of Liptakov's (LoL) does it perfectly so maybe inspire from his schèmes, pictures, videos.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2894
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | Why TACN?? the perchlorate is so much cooler.
Can someone please try to grow a large crystal of the TACP, it is so pretty, plus I would like to measure the det properties of a monocrystalline
explosive. And they are so pretty. Any tips on how to do this other than cooling the solution slowly  |
Why not?  ... ...
Almost everybody gets his first hands onto TACuN...the process is cheap, easy and beautifull.
Some people don't have easy access to ammonium perchlorate (AP), copper perchlorate or perchloric acid...to be able to make TACuP...and making those
OTC is more labour intensive and cost more than obtaining nitrates or ammonium nitrate (AN)...
*********************************
About your monocrystalline TACuP...
Instead of slow cooling...you may:
1°) Make a saturated solution of TACuP and introduce a seeding cristal...then allow for slow evaporation...like common large Crystal growing
techniques...
2°) Make a saturated solution of TACuP with a seeding cristal into it (into a tiny recipient) and allow for a slow migration of solvent (by natural
evapo-condensation) into a closed large recipient holding the volatile solvent (methanol or better ethanol or even better isopropanol).
The volatile solvent will evaporate into the closed volyme condensate into the saturated TACuP solution (the TACuP solution will evaporate some water
into the surrounding and thus concentrate a little)...so you get two effects into the solution...a slight concentration and a solubility drop (induced
by the solvent)...this results into crystallization that is easier onto the already present seeding crystal...the later will thus grow.
Usually the solvent condensate at the surface of the liquid into the inner tiny recipient...you thus observe a concentration gradient (upper layer is
paler even colorless-clear at a certain stage) while the bottom is deep blue like initial TACuP...during crystallization the amount of dissolved TACuP
diminishes and as a consequence the solution becomes paler and paler (the blue color fades).
3°) You work with a saturated TACuN solution and you introduce slowly drop by drop a saturated AP solution into the media...until it becomes slighly
milky/turbid ==> then you add a few drops of water to make it clear again (to be on the edge of precipitation of TACuP.
Again you put a seeding Crystal of TACuP (suspended into the fluid), and you allow for slow introduction of saturated AP solution drop by drop under
slow agitation (1 drop per 5 minutes)...
TACuP is less soluble than TACuN and hence it will precipitate and this process is easier onto the already present seeding Crystal...the solution of
TACuN will become paler and paler...but it needs to be refreshed somehow also slowly.
At the end into solution you will have AN, TACuN and a little TACuP and AP...
This later process is less convenient that the previously exposed.
4°) You need 3 recipients...and tubings (see drawing below)
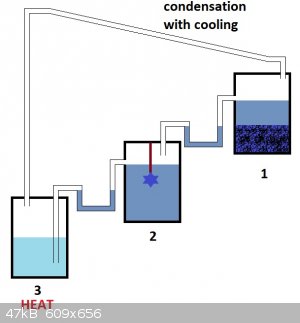
-The nr 1 contains crystals of TACuP and a saturated solution of TACuP
-The nr 2 contains a seeding crystal suspended into saturated solution of TACup
-The nr 3 contains initially water...but it will enrich itself into TACuP of lower concentration than the saturated solution.
The third recipient may be slighly heated to ensure evaporation of water that will condensate into the first..
When this happens the same volume of saturated TACuP solution will be displaced into the second recipient holding a seeding crystal...what will
grow...and depress concentration of the saturated TACuP soluation a little...
When this happens the same volume of less saturated TACuP solution will fall into the third recipient.
From time to time when concentration into the third recipient becomes too saturated it can be refreshed by new water and the initial content
evaporated to regain TACuP crystals recyclable into the first recipient...
The syphons needs to be amorced by introducing some saturated fluid into them...
In principle no heat is needed (since vapor pressure of the third recipient will be higher than the first as long as there is a difference in
concentration between recipient 3 (dilluted) and 1 (saturated)) but this may help the process to be a little faster...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
2 |