| Pages:
1
2 |
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Steam pressure chamber for 1000C
I want to make a chamber to hold water vapour at 1000C (one thousand C) at less than 50 psi.
Having attempted to read up on the subject I reckon I need hastelloy or inconel 625 to make the actual chamber. I am sure that there are other alloys
but they seem to be the most common and that is their most common names.
The chamber will sit in a furnace at around 1000C and will be approx. 6 inches long by 2 inches in diameter (a pipe).
Wall thickness about 6mm thick?
One end will probably be welded shut with a black plate and the other end will have an door of sorts.
The door will consist of a blank plate with four nuts and bolts holding it shut with the bolts welded to the pipe.
The end cap and pipe end will have to be ground flat for a seal.
The device will run for hours at a time.
Is there anything I can use for a door seal?
Can anyone advise me on this as I have not a clue as to whether or not this will work.
Hastelloy is not easy to get cheap. I was hopeing to get some end cuts on ebay but they are not to be had though that could change.
eg.
http://www.ebay.com/itm/Galigher-Hastelloy-Sleeve-58253/2709...
Cheers,
Yob
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
1000°C is not possible at 50 psi. Saturated steam pressure at 374°C is 3206 psia.
Check the steam tables for saturated steam.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Quote: Originally posted by Magpie  | 1000°C is not possible at 50 psi. Saturated steam pressure at 374°C is 3206 psia.
Check the steam tables for saturated steam.
|
1000C steam is possible at any pressure. It's just a matter of having the right amount of water in the chamber. I have no idea why anyone would want
to do that (high temp steam at low pressure), but it can obviously be done.
You'd have to evacuate air from the chamber before heating it because the air would make substantial pressure itself . Just the right amount of water
in a fairly hard vacuum and when heated you'll get 50PSI at 1000C.
I would ordinarily be quite reluctant to contradict Magpie on matters such as this, however this seems pretty clear-cut.
EDIT: If you just heat water to 1000C in a chamber with a 50PSI blowoff valve you'd get almost the same result since all that vented steam would purge
nearly all of the air along with it. Don't know where you could find a blowoff that functions at those temperatures and pressures though.
[Edited on 12-1-2017 by Maroboduus]
EDIT: Any pressure below the threshold for going supercritical, that is.
[Edited on 12-1-2017 by Maroboduus]
[Edited on 12-1-2017 by Maroboduus]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have to agree that water can be evaporated to a gas and held at 1000°C, or any higher temperature, for that matter, at 50psi. But it won't be
saturated steam and there will be no liquid water in the same location.
edit: For 1000°C I think you are going to have to use a graphite gasket. Or, possibly a screwed cap with lapped mating surfaces for a
metal-on-metal pressure fit might work.
[Edited on 12-1-2017 by Magpie]
[Edited on 12-1-2017 by Magpie]
[Edited on 12-1-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | I have to agree that water can be evaporated to a gas and held at 1000°C, or any higher temperature, for that matter, at 50psi. But it won't be
saturated steam and there will be no liquid water in the same location.
edit: For 1000°C I think you are going to have to use a graphite gasket. Or, possibly a screwed cap with lapped mating surfaces for a
metal-on-metal pressure fit might work.
[Edited on 12-1-2017 by Magpie]
[Edited on 12-1-2017 by Magpie]
[Edited on 12-1-2017 by Magpie] |
At 1000 C there won't be any liquid water regardless of the pressure.
https://en.wikipedia.org/wiki/Critical_point_(thermodynamics)
Nobody asked about saturated steam, did they?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
No, that was my own misinterpretation.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have to say the interesting question here is "why?".
Why would anyone want thousand degree steam?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
But if you heat a container to 1000 degrees, it will not contain any real amount of steam, as it would vent out immediately, due to the vapor
pressure, and there would not be any real amount of steam left. Temperatures much lower that that are used to dry things, so all water vapor will be
driven off well before 1000 degrees, unless the container is sealed, and then it will just explode. Without special pressure equipment, this makes no
sense.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was reading some ranges for power stations.
"For a Super/Ultra critical steam turbine (3 to 4 Pressures/Sections , as high as 1000 Mw) - HP inlet Pressure/Temp - 235/285 bar and 570/620 Deg
C."
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
It''s really quite simple, just add a pressure regulator. It would have to be mounted outside the furnace, but that's not really a big deal.
Expect to do most of the leg work yourself, 1000°C is outside most peoples experience and comfort zone. I would assume that almost any of the
nickel-based superalloys would work, you just have to see what you can get hold of.
For calculating the minimum wall thickness, here's a useful page:
http://www.amesweb.info/StressStrainTransformations/StressIn...
We're not banging rocks together here. We know how to put a man back together.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fulmen  |
It''s really quite simple, just add a pressure regulator. It would have to be mounted outside the furnace, but that's not really a big deal.
|
The regulated would have to be at the same temperature as the pressure vessel to prevent water condensing in it.
On a different point: like Dr Bob I am curious as to what the OP is trying to do.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  | Quote: Originally posted by Fulmen  |
It''s really quite simple, just add a pressure regulator. It would have to be mounted outside the furnace, but that's not really a big deal.
|
The regulated would have to be at the same temperature as the pressure vessel to prevent water condensing in it.
On a different point: like Dr Bob I am curious as to what the OP is trying to do.
|
No, it would have to be hot, but not that hot. As long as it's above the boiling point of water at 50PSI it's not going to be an issue.
There are also other tricks you can use.
Is there anyone who doesn't wonder what the OP's objective is?
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Yes Unionised is correct. I checked about 135C for about 3 bar so not too difficult.
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
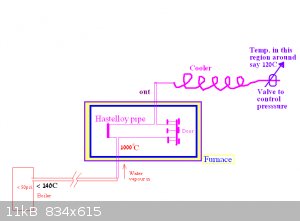
Thanks alot for all the replys.
Hope the application does not sound too boring.
I am conveting iron to magnetite as per this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=71009
I thought it might be useful to up the pressure a bit if it is not too difficult, not that I can say it will be any advantage.
See diagram.
"No, it would have to be hot, but not that hot. As long as it's above the boiling point of water at 50PSI it's not going to be an issue.
There are also other tricks you can use."
What other tricks could you use to regulate the pressure?
If I used graphite as a gasket would it not oxidize at 1000C in the presence of water vapour?
Cheers,
Yob
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
50 psi is not a very high pressure but 1000C is a quite high temperature. I would use steel, use lapped mating surfaces, forget pressure regulation.
Calculate the amount of water needed and assume you reach pressure at temperature.
Don't use a standard flange (too large) but instead use a screwed cap (internal threads). I hope you have a large budget.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Quote: Originally posted by wg48  | | The regulated would have to be at the same temperature as the pressure vessel to prevent water condensing in it. |
Not really. So what if there is condensed water in the system? Start with a excess of water, as it evaporates it will bleed off through the regulator.
If you want simple regulation you could try a simple weighted lid (pressure cooker style), but I fear you will have problems maintaining a perfect
seal.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm going to reverse myself and say that I like Fulmen's pressure regulator. This would be a good safety device and eliminate the need for
hydrostatic testing.
You are building a pressure vessel. The high temperature will derate the normal strength of the metal. This must be taken into account during
design.
Will the pressure drop during the reaction to form magnetite? Does this matter?
My household water pressure is 65 psi. Water line connections at the sinks are metal-to-metal compression sealed.
Nuclear grade graphite gasketing good for 1000C is available.
[Edited on 13-1-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Personally i would reverse your setup and build the furnace inside the pressure chamber. That way you could use cheap, readily available materials
(like steel) for the chamber as it would be at lower temperature- say 200C to prevent condensation.
This also greatly expands the range of materials you can use for the 1000C reaction chamber, as it no longer has to resist any pressure, or be sealed
tight.
In my opinion, trying to build a pressure chamber to withstand 50psi at 1000C is quite a challenge.
And ask yourself this: if iron reacts with water at 1000C, what happens to Hastelloy? Or any other metal you might choose?
[Edited on 13-1-2017 by Twospoons]
[Edited on 13-1-2017 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am not understanding the why of this project.
Unless you have an enormous volume the actual quantity of steam will be small. Are you sure that you cannot achieve your goal with an HHO torch?
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | 50 psi is not a very high pressure but 1000C is a quite high temperature. I would use steel, use lapped mating surfaces, forget pressure regulation.
Calculate the amount of water needed and assume you reach pressure at temperature.
Don't use a standard flange (too large) but instead use a screwed cap (internal threads). I hope you have a large budget. |
Use ordinary steel?
I will be placing pieces of ordinary steel into the (inert) chamber and they will be converted to magnetite by the action of the hot water vapour.
This takes about 12 hours of action at atmospheric pressure (a stream of water vapour going into a simple brick furnace heated with nichrome wire) to
convert a piece of steel that is about 3mm thick. Hydrogen produced must be kept sweped away.
I cannot use ordinary steel in my pressurised reaction chamber where the temperature is high as it will simple react with the water vapor and turn
into magnetite.
I can use ordinary steel or perhaps more ordinary stainless steels outside the furnace where temperatures are low.
I was hopeing a simple waited or spring loaded valve on the very end of the outlet pipe would suffice as a valve. Simple, cheap and safe.
The graphite seals from the nuclear industry would cost an arm and a leg I would imagine.
There are lots of graphite seals for exhausts available but they will not work in an oxidizing atmosphere at 1000C. Exhausts fumes would be reducing
atmospheres.
I would use a screw cap on the reaction chamber/tube if I can get a piece of hastelloy (etc) with threads on it and an end cap but that is unlikely.
It would cost too much to have a threaded pipe and end cap (hastelloy made up.
I might get lucky and get something on ebay. Here's an end cap of sorts.
http://www.ebay.com/itm/Dixon-1-Hastelloy-Cam-Lok-Couplers-H...
Its a bt too small (one inch dia).
Now all I need is the threaded pipe!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't see how that coupler would work - it's open on both ends.
Are you going to have to pump in water to maintain pressure and vent H2?
edit: I see from your sketch that that is what your boiler will do.
Please give us the chemical reaction for this process.
[Edited on 14-1-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by yobbo II  | Thanks alot for all the replys.
Hope the application does not sound too boring.
I am conveting iron to magnetite as per this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=71009
I thought it might be useful to up the pressure a bit if it is not too difficult, not that I can say it will be any advantage.
See diagram.
"No, it would have to be hot, but not that hot. As long as it's above the boiling point of water at 50PSI it's not going to be an issue.
There are also other tricks you can use."
What other tricks could you use to regulate the pressure?
If I used graphite as a gasket would it not oxidize at 1000C in the presence of water vapour?
Cheers,
Yob
|
That's interesting to know.
I rather suspect it means that your system won't work- because there's an equilibrium between steam, hydrogen, iron and its oxides.
Get some sort of container, put iron in it, heat it to 1000C, blow steam through it from some sort of steam generator to flush out the hydrogen
produced.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I see. You will need a continuous stream of water vapor, luckily this shouldn't be hard to achieve. A small boiler running at operating pressure
should be simple to make, and as long as you have a continuous gas feed a weighted valve should work fine.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This isn't a complicated process- it's been around for a couple of hundred years and used to be common enough experiment to get described in books as
a preparation method for hydrogen.
the "typical" vessel back then was a gun barrel and it was heated to red hot.
Any bit of modern thick walled steel pipe would do the job
https://books.google.co.uk/books?id=vBpkAAAAcAAJ&pg=PA12...
https://books.google.co.uk/books?id=-ucTAAAAQAAJ&pg=RA1-...
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
1000°C at 50psi is only possible if some of the steam is allowed to vent (constantly). But it'd be hard to regulate that.
[Edited on 15-1-2017 by xfusion44]
|
|
|
| Pages:
1
2 |