yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
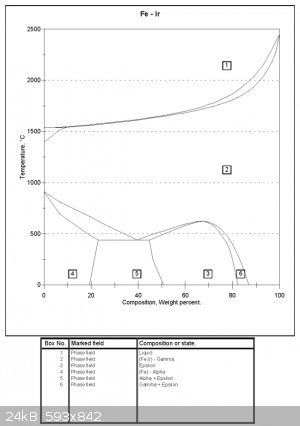
Iron-Iridium phase diagram
There is an Iron-Iridium phase diagram here
http://www.pgmdatabase.com/jmpgm/index.jsp?database=cesdatab...
Don't know much about phase diagrams. Can anyone tell me what you get in region 4. The diagram key states it's alpha iron, but there has to be some Ir
there too (say at around 2% Ir, 98% Fe). Perhaps the system has not been studied enough and they simply do not know what phase the Ir is in around
those percentages?
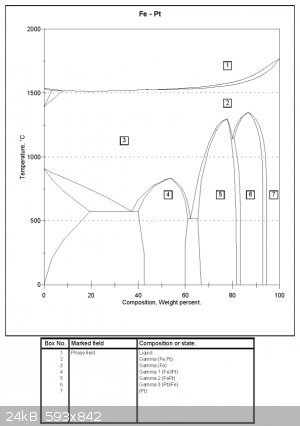
Same with Pt-Fe here
http://www.pgmdatabase.com/jmpgm/index.jsp?database=cesdatab...
What do you get around 2% Pt at low temperatures? It is simply unknown?
The links above do not work correctly for some reason.
Both diagrams are attached
[Edited on 25-10-2016 by yobbo II]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
It's alpha-iron with dissolved iridium in it. There isn't enough iridium to change the crystal structure at this temperature.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Exactly. Ferrite (alpha iron) doesn't indicate composition, only crystal structure. Here's more on iron allotropes:
https://en.wikipedia.org/wiki/Allotropes_of_iron
We're not banging rocks together here. We know how to put a man back together.
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Thanks for replys.
So I simply have a solid solution of Iron (alpha crystal structure) + Ir at low percentages of Ir and low temperatures.
What about the Pt-Iron diagram. What do you get in the two areas associated with low % of Pt and low temperatures?
What is Epsilon Iron? According to the link Fulmen provided it only occures at high pressure (for Iron + Carbon anyways).
If I melt Iron and add one percentage Pt and cool what will I get?
I will probably be cooling over a period of some seconds though I could heat up again I suppose.
I am trying to get an alloy of Iron and Pt around 1 or 2 % Pt where the Pt is in solid solution with the Iron. I don't want the Pt to be isolated in
areas of a different phase in the alloy. (if that makes sense)
|
|
|
j_sum1
Administrator
       
Posts: 6323
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
The designations alpha, beta etc are specific to the phase diagram. Which basically means that an alloy designated epsilon on the Fe-Ir diagram has
nothing to do with "epsilon" on another Fe phase diagram. That said, some designations have become standardised. Alpha iron is synonymous with
ferrite and occurs at high Fe concentrations on all Fe phase diagrams. Similarly gamma is synonymous with austenite.
That seems clear as mud. I won't bother rewording. On your particular diagram, epsilon refers to an alloy of Pt with 20-50% Fe. I have no idea what
its crystal structure might be.
As for your Fe-2%Pt alloy, the diagram indicates that it is easily possible at high temperatures. At low temperatures the solubility of Pt in Fe is
less and the diagram (if accurate) suggests that at 0°C you will have a two phase system with mostly ferrite and some grains of Fe3Pt. The latter
quite probably exists as a eutectoid -- a microstructure where there is intimate layering of two phases caused as the local concentration fluctuates
while the crystals are growing.
But what you need to remember is that these are equilibrium diagrams. At low temperatures (eg, room temperature) you are not going to get
recrystallisation. Alloys typically exist in a metastable configuration that reflects their thermal history. In this particular case you are likely
to get your desired result by heating to 250°C and quenching. A rapid temperature drop will not allow time for the Fe3Pt to come out of solution as
a separate phase. The only way to confirm this however is through microscopy.
Is there any particular reason why you need a single phase?
|
|
|