| Pages:
1
2
3
4 |
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Valorizing the Glycerol from Biodiesel Production
Hello everyone!
This is a spin-off from Chemoleo's thread regarding the production of biodiesel. It became apparent that we will soon have more glycerol than we know
what to do with.
Here, we shall address the issue of valorizing, or adding value to the glycerol produced during the manufacture of biodiesel. Other than nitration, I
think that just about anything is fair-game.
So far, we have discussed fuel use, and why this is not such a good idea (acrolein, polymer fouling), and, ahem, laxatives.
This, however segues neatly into chemistry and biochemistry that can be performed to turn glycerol into more valuable material. Granted, acrolein is
*extremely* toxic, but it is used as an industrial feedstock of value.
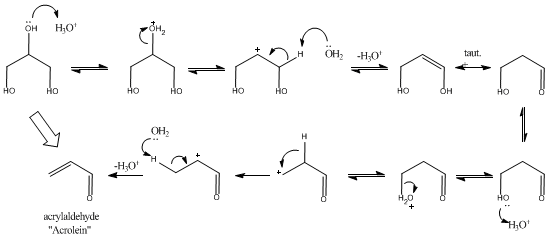
Attached is a mechanism outlining the E1 dehydration of glycerol under anhydrous conditions (hard to avoid at the boiling point of glycerol!). My
apologies if there is a mistake in it, but I just whipped it out for this.
I have also noticed that vinyl dioxolanes result from the acetal formed with glycerol and product acrolein.
For the Biochemists amoung us, an interesting article with full text:
"METABOLISM OF GLYCEROL BY AN ACROLEIN-FORMING LACTO-BACILLUS"
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=27...
How about 1,3-propanediol?
Happy, er, greasling?
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Esters with other things oughta make it a better fuel... how about...alkyl carboxylic acids, possibly long chain?
Oh...er...nevermind.

Esters have a lot of oxygen in them, but how about ethers with alkyl-ols, how hard are those to stick to glycerin? (Probably not economical, but
we're not minding that, huh?)
Tim
[Edited on 11-15-2006 by 12AX7]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have worked with acrolein and I don't recommend it. They weren't kidding when they sugested it as a war gas.
Can ordinary yeast ferment glycerine to anything- alcohol would be useful but dull.
Come to think of it, what do mamals metabolise it to- would it be useful as animal food?
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Lube? It IS one of the main components in KY jelly AFAIK. Not only will we have cheap fuel from biodiesel, we'll be happy when not driving our cars
as well 
Dynamite and lube are really the only two easy uses I see coming from it.
Although, perhaps acrolein could be oxidized to acrylic acid and used as a starting material to make plastics? Does anyone know how easily such a
reaction occurs, and how easy acrylic acid is to form an ester?
I guess one could react with a strong base to give a salt of acrylic acid and allyl alcohol, since if we have glycerin coming out of our ears from
biodiesal production, we can afford to lose half of it to allyl alcohol, which I'm sure has some good uses as well.
Unfortunately we are still going through acrolien at one point in the procedure.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Good evening!
Well, glycerine is an important side product from the ethanol fermentation of molasses using yeast. From what I understand, the ethanol fermentation
is performed first, and then the osmotic pressure is kicked up and the stillage refermented to yield lots of glycerol (a process that led to a glut in
the existing market). A full paper is given here:
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=168312...
Also, USDA (and Rice, it looks like) is looking at further fermentation of glycerol to yield, among other things, succinic acid (This is also the
objective furthered by DOE). Please, see:
http://www.ars.usda.gov/research/publications/Publications.h...
As for nutritive value, it appears to be negligible as the body seems to quickly metabolize what it needs to make triacylglycerides and phospholipids;
some gets shunted around via the glycerol phosphate shuttle to yield ATP in mitochondria. The rest is eliminated in urine and the excretory half-life
is rather short, viz. 30min. For more information, see the pharmacokinetics subheading here:
http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrug...
See also, glycolysis, where glycerol exists as an intermediate cycling from dihydroxyacetone. This is a small part of the lactic acid, or Cori cycle.
Oh yes, acrolein is foul. Even with an *efficient* hood, you can tell that something is bothering you.
Yes, acrylic acid and allyl alcohol have good industrial value. This is mostly for the manufature of acrylic polymers, etc., viz. poly-acrylic acid
(fibers). It too stinks, and has been known to destructively polymerize under temperature gradient even when cold. I retrieved some from a freezer
once, and read the container that said specifically, do not freeze--local heating can cause destructive polymerization... I realized that my fingers
were locally heating it, and placed it *carefully* into the hood. Nothing bad happened. It warmed up and I went on with life. Stinky though.
It looks like it would probably oxidize in air, faster with a catalyst (but, that double bond is just looking for a reason to polymerize). Maybe
addition of a heterogenous inhibitor like Cu filings would prevent this whilst enabling the oxidation?
'evening all,
O3
[Edited on 16-11-2006 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Glycerol retains moisture. Wouldn't it be useful for agriculture if mixed to soil? Not for the water itself, but as a soil "softener".
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Do you mean "easier to dig"? I know that they sell special polymers that can hold many times their weight in water for use in soil.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Yes, that is the idea, but I was thinking about farming, not flower pots.
Can't glycerol be polymerized?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hmm. That might work, but would take a lot of glycerol. Glycerol is water soluble--wouldn't it eventually leach out during rains? What would be the
potential environmental impact of the application of glycerol on this scale (it will definitely increase COD and likely, BOD as well)?
It can be step polymerized via esterification, I have done this with citric acid (with additional H2SO4 catalyst). It gets very thick, and you can
pull fibers from it...But, it is hygroscopic and unstable (it dissolves and depolymerizes in contact with moist air, quickly). This could result from
either remaining catalyst in the polymer (which is, itself hygroscopic) and/or relatively low molecular weight (99.+% conversion is kinetically
required for HMW polycondensation).
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Adding three halide atoms to glycerol should be easy. Trichloropropane is a nasty pesticide.
triiodopropane - 13 hits on google
tribromopropane - 564 hits
Any use for that?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Happy Thanksgiving!
The triiodo species referred to online are predominantly 1,1,1; 1,1,2; and 1,2,2. This implies radical halogenation and/or addition across a double
bond, not nucleophilic substitution (as we would see with an alcohol). Simply adding HCl (conc) to glycerol will yield via SN1 (mostly) the most
stable (in this case 2°) carbocation which will then be attacked by X- (X=F<Cl<Br<I, in aqueous media) to yield mostly
2-iodo-1,3-dihydroxypropane. This might be interesting as the skies-the-limit on what you can do with a leaving group that is as good as I (very soft
nucleophile). In order to carry out the SN2 required on the 1 and 3 hydroxyls, a polar aprotic solvent (acetone is a good one for this) will be
preferred.
This is of course, assuming that the glycerol stays functionally intact thoughout the procedure.
At either rate, the idea is I think, is good-leading to further discussion, viz. where can we go from, say, 2-iodo-1,3-dihydroxypropane.
[edit] that's weird, the text window is correct, but the preview and post seem to cut off: - (X=F<Cl<Br<I, in aqueous media) to yield mostly
2-iodo-1,3-dihydroxypropane. This might be interesting as the skies-the-limit on what you can do with a leaving group that is as good as I (very soft
nucleophile). In order to carry out the SN2 required on the 1 and 3 hydroxyls, a polar aprotic solvent (acetone is a good one for this) will be
preferred.
[edit] let's try that again: to yield mostly 2-iodo-1,3-dihydroxypropane. This might be interesting as the skies-the-limit on what you can do with a
leaving group that is as good as I (very soft nucleophile). In order to carry out the SN2 required on the 1 and 3 hydroxyls, a polar aprotic solvent
(acetone is a good one for this) will be preferred. *the part the post did not like was an order of nucleophilicity in water where F is least and I is
greatest which used "less-than" symbols.
Gobble Gobble,
O3
[Edited on 23-11-2006 by Ozone]
[Edited on 23-11-2006 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by Ozone
X- (X = F [lesser than] Cl [lesser than] Br [lesser than] I, in aqueous media) |
Nothing to disable HTML parsing. "Lesser than" begins an HTML object...
Tim
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks!
I narrowed that down as the cause, but was not quite sure why!
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Ozone also doesn't seem to affect glycerol. That is sad, because if we could cheaply oxidize one or two of those OH groups to carboxilic acids there
would be a huge crosslinking due to ester formations among molecules, wouldn't it?
I tried to add some acetic + sulfuric acids to glycerol, hoping to get the thickening you mentioned with citric acid, but nothing happened.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Tacho,
It's got to be pretty hot! Regulate the temperature at ~130-150°C and try first, a 1:1 molar equivalence (thick paste). Add the citric acid (I use
anhydrous, but at these temperatures, it should be OK), then the glycerol. Begin heating, stirring with a glass rod. At about 60°C the mixture should
"loosen-up". Add 1 drop of H2SO4 (conc.). Magnetic stirring can be done from here until the mixture reaches conversion sufficient to stop the bar. Try
removing a drop with a glass rod, cooling slightly, touching it to the bottom of a beaker; try to pull a thread. It should do this. The fiber will
then get wet and dissolve . .
I am playing with this to try and get something that is not so hygroscopic. I've started taking photos, and should be able to post some soon.
Good luck,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Tacho
I tried to add some acetic + sulfuric acids to glycerol, hoping to get the thickening you mentioned with citric acid, but nothing happened.
|
You won't get thickening with acetic acid. Citric acid is a tribasic acid, it forms polymers with polyols. Acetic acid is monobasic, you just get
esters of acetic acid, although in this case it would have the trival name "glycerol acetate" with 2 or 3 acetates combined with each gycerol
(depending on how hard you wack the reation mix).
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
IIRC glycerol through a hot tube with zinc catalyst produces predominantly pyruvic acid, but I might be wrong.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Hydrogen or Syn Gas Production from Glycerol Using Pyrolysis and Steam Gasification Processes
Valliyappan, Thiruchitrambalam
Thesis Degree- Master of Science, Department Chemical Engineering
http://library2.usask.ca/theses/available/etd-01042005-14033...
Abstract
Glycerol is a waste by-product obtained during the production of biodiesel. Biodiesel is one of the alternative fuels used to meet our energy
requirements and also carbon dioxide emission is much lesser when compared to regular diesel fuel. Biodiesel and glycerol are produced from the
transesterification of vegetable oils and fats with alcohol in the presence of a catalyst. About 10 wt% of vegetable oil is converted into glycerol
during the transesterification process. An increase in biodiesel production would decrease the world market price of glycerol. The objective of this
work is to produce value added products such as hydrogen or syn gas and medium heating value gas from waste glycerol using pyrolysis and steam
gasification processes.
Pyrolysis and steam gasification of glycerol reactions was carried out in an Inconel®, tubular, fixed bed down-flow reactor at atmospheric
pressure. The effects of carrier gas flow rate (30mL/min-70mL/min), temperature (650oC-800oC) and different particle diameter of different packing
material (quartz - 0.21-0.35mm to 3-4mm; silicon carbide – 0.15 to 1mm; Ottawa sand – 0.21-0.35mm to 1.0-1.15mm) on the product yield, product gas
volume, composition and calorific value were studied for the pyrolysis reactions. An increase in carrier gas flow rate did not have a significant
effect on syn gas production at 800oC with quartz chips diameter of 3-4mm. However, total gas yield increased from 65 to 72wt% and liquid yield
decreased from 30.7 to 19.3wt% when carrier gas flow rate decreased from 70 to 30mL/min. An increase in reaction temperature, increased the gas
product yield from 27.5 to 68wt% and hydrogen yield from 17 to 48.6mol%. Also, syn gas production increased from 70 to 93 mol%. A change in particle
size of the packing material had a significant increase in the gas yield and hydrogen gas composition. Therefore, pyrolysis reaction at 800oC,
50mL/min of nitrogen and quartz particle diameter of 0.21-0.35mm were optimum reaction parameter values that maximise the gas product yield (71wt%),
hydrogen yield (55.4mol%), syn gas yield (93mol%) and volume of product gas (1.32L/g of glycerol). The net energy recovered at this condition was
111.18 kJ/mol of glycerol fed. However, the maximum heating value of product gas (21.35 MJ/m3) was obtained at 650oC, 50mL/min of nitrogen and with a
quartz packing with particle diameter of 3-4mm.
The steam gasification of glycerol was carried out at 800oC, with two different packing materials (0.21-0.35mm diameter of quartz and 0.15mm of
silicon carbide) by changing the steam to glycerol weight ratio from 0:100 to 50:50. The addition of steam to glycerol increased the hydrogen yield
from 55.4 to 64mol% and volume of the product gas from 1.32L/g for pyrolysis to 1.71L/g of glycerol. When a steam to glycerol weight ratio of 50:50
used for the gasification reaction, the glycerol was completely converted to gas and char. Optimum conditions to maximize the volume of the product
gas (1.71L/g), gas yield of 94wt% and hydrogen yield of 58mol% were 800oC, 0.21-0.35mm diameter of quartz as a packing material and steam to glycerol
weight ratio of 50:50. Syn gas yield and calorific value of the product gas at this condition was 92mol% and 13.5MJ/m3, respectively. The net energy
recovered at this condition was 117.19 kJ/mol of glycerol fed.
The steam gasification of crude glycerol was carried out at 800oC, quartz size of 0.21-0.35mm as a packing material over the range of steam to
crude glycerol weight ratio from 7.5:92.5 to 50:50. Gasification reaction with steam to glycerol weight ratio of 50:50 was the optimum condition to
produce high yield of product gas (91.1wt%), volume of gas (1.57L/g of glycerol and methanol), hydrogen (59.1mol%) and syn gas (79.1mol%). However,
the calorific value of the product gas did not change significantly by increasing the steam to glycerol weight ratio.
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thank you, Solo.
The only problem with most Fisher-Tropsch and friends is the cost of energy (and, in the first case, carrier--N2 at rates required at industrial scale
can rapidly offset the product value, particularly if the product(s) need be transported). In the US, most industrial energy (steam) is derived from
natural gas (NG) powered boilers. NG has been floating between 4 and $12 US per mcf,viz. unless you are using CO or H2 directly in a process
(insertion chemistries, hydrogenation) the price vs. NG will not likely work in your favor; this is why the NH3 industries in the US have gone
four-legs-up-the cost of fueling the process with NG was too high for profit on operation to be achievable.
However  , These processes, particularly the hydrocracking, *might* be feasible at
a sugar mill which is powered almost entirely on bagasse, which is free. The vertical integration of this mill with a nearby end user would be
required, however. , These processes, particularly the hydrocracking, *might* be feasible at
a sugar mill which is powered almost entirely on bagasse, which is free. The vertical integration of this mill with a nearby end user would be
required, however.
Organikum, do you have a reference for the Zn/pyruvate reaction (this would be something I would try)?
I have found some methods (in the literature) whereby glycerol is treated with Zn/HCl to yield 1,3-propanediol (I will try to get the refs. tomorrow;
I have them in the work-lab). Presumably, this must go through the dihydroxyacetone intermediate which then undergoes Clemmeson-type reduction to
yield the product; it must take place in dilute solutions to prevent the facile dehydration of the 2° hydroxyl?
Most recently, I have tried the polycondensation in ionic fluids*. Something seems to happen (viscosity goes way up) in 1-ethyl-3-methylimidazolyl
methanesulfonate, but the products are difficult to isolate, viz. ionic liquids seem to solvate fricking everything (except for solvents that I am
trying to avoid in a "green" synthesis. Bummer. I did get some white crystalline ppt from a sample dissolved in water+Na2CO3 and partitioned with
MeOH. I suspect this is a "worthless" salt. I'll filter it tomorrow and run it by some tests.
*these things have virtually no vapor pressure, and considerbale dipoles. As such they can be flash heated to great temperatures very quickly using a
microwave! Watch it though, the violent boiling results from the flash boiling of the water evolved during the esterification (which is good, because
it cannot reverse the reaction and bad, as it is a pain-in-the-ass to clean the stuff up when it spews everywhere).
The burning let's you know it's working , ,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Jome
Hazard to Others
  
Posts: 154
Registered: 10-6-2004
Location: Soutwest sweden
Member Is Offline
Mood: desiccated
|
|
Most likely if biodiesel became economically feasible it would be based on algae-oil, something like caulerpa taxifolia (yes, killer algae) that kind
of source beats everything else by a factor of ten.
Ideally, other parts of the plant would suffice both to be turned into CO and then the spare energy from burning the rest would be enough to make this
into the methanol needed in the BD synthesis.
Therefore, the other chemical roles of old-time petroleum should be what we are after, such as lubricants, waxes, starting materials
for plastics... Could perhaps a benzene ring be made from glycerol? That'd be immensely useful since most of those today come from petroleum.
[Edited on 29-11-2006 by Jome]
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
If pyruvic acid can be turned into ethanol by yeast and glycerol is substantially cheaper than ethanol, then the glycerol-zinc path sounds very
interesting.
Very interesting indeed. Thanks Organikum.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Good day all!
Jome,
That is more or less what I am after--The means to produce chemical feedstocks from sources other than petroleum. For benzene rings, though, I would
go after the lignin byproducts derived from pretreatment of biomass for cellulosic ethanol, or, from the paper industry. The average US sugar mill,
for example, would produce ~70 tons (2000lb/ton) per day, if it were to be making cellulosic ethanol.
What is the mass balence for this algae you describe? Removing lots of water requires dealing with much latent heat and larger energy expenses. Still,
good idea! Recycling the unused portions for fuel is always a winner (until you figure out something more lucrative to do with it)!
Attached are some pictures of the poly-glycerolcitrate amd the crystals I got from the crazy ionic liquid.
Cheers,
O3
[Edited on 30-11-2006 by Ozone]

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Thank you not_important, I can see why I didn't get any thickening. I'll try oxalic acic. It will probably generate long chain polymers.
Ozone, removing water is not an issue in hot countries where there is plenty of sun. Nice pictures btw, thanks.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
For a truly off-the-wall idea, how about using it in high altitude smoke machines, generating aerosols to combat global warming. And make pretty
sunsets.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
I was just thinking.... Is it possible to oxidize the acrolein into glyoxal? The structures look pretty similar except glyoxal has an extra oxygen
atom.
|
|
|
| Pages:
1
2
3
4 |