| Pages:
1
2
3
4 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Succinic Acid
Attempt at the preparation of succinic acid via hydrogenation of fumaric acid, a failure
1. Introduction
This report describes an attempt to make succinic acid by the hydrogenation of fumaric acid:
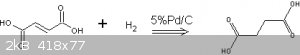
The purpose of this experiment was not only to obtain succinic acid but to evaluate the apparatus and gain further hydrogenation experience using a
metal catalyst.
The procedure used was based on that for preparing succinic acid in Vogel (ref 1). Although the Vogel procedure specifies maleic acid as precursor
Ullmann (ref 2) indicates that it can also be made from fumaric acid, its geometric isomer. However, I could not find any procedure specifying
fumaric acid as precursor. The apparatus used was essentially that in Pavia (ref 3) for the preparation of methyl stearate from methyl oleate via
hydrogenation.
2. Reagents
6.9g fumaric acid
1.4g 5% Pd/C
100ml absolute ethanol
100ml 6M H2SO4
14.4g mossy Zn
10ml mineral oil
CaCl2 granules

Reagents
3. Equipment
250ml 2-neck RBF, 19/22
125ml Buchner funnel flask
50mL p-e funnel
CaCl2 guard tube
6”test tube with tublature
6mm glass tubing
¼” ID latex tubing
centrifuge w/4 test tubes
5” evaporating dish
magnetic stir bars
magnetic stirrer
magnetic stirrer-hotplate
2ea 1-hole #2 rubber stoppers
19/22 adapters w/tublature
7cm Buchner filter
4. Safety
The 5%Pd/C can catalyse a violent combustion at room temperature. Therefore, great care must be exercised when adding it in the presence of
combustible vapors and air. When loading the light fluffy Pd/C it is important to get it fully submerged in the solvent, avoiding leaving any
deposits on the interior walls of the reaction vessel.
During my only other hydrogenation, in a school lab, methyl oleate was dissolved in methanol in the reaction vessel. As I was loading the 10% Pd/C
into the vessel there was a small but loud explosion. At that time I gained a healthy respect for metal catalysts.
5. Procedure
a. hydrogenation
The hydrogenation apparatus is shown in the picture below:

The 6” test tube bubbler was loaded with enough mineral oil to provide ~1” of submergence for the H2 outlet glass tube. CaCl2 granules were loaded
into the guard tube. This was intended to insure that the hydrogen supplied to the reaction vessel was dry. An initial 5.2g of mossy Zn was placed
in the Buchner flask along with a stir bar. 50mL of 6M H2SO4 was placed in the p-e funnel. The system was purged with 1.5L of argon. 100mL of
absolute ethanol was added to the 250-mL reaction flask along with an oval stir bar. 6.9g of fumaric acid was added to the reaction flask. Stirring
was started but not all of the fumaric acid dissolved, as was anticipated. The 6mm glass sparge tube opening was situated just above the rapidly
turning stir bar. 1.4g of 5% Pd/C was carefully added to the reaction flask using a paper funnel. The oil bath was heated to 40-50°C to promote
solubility of the fumaric/succinic acids.
The 6M H2SO4 was slowly dripped onto the stirring mossy Zn to effect an H2 effluent rate of 2-3 bubbles/s at the bubbler, per Pavia. The Zn and acid
were replenished as necessary to provide a hydrogenation time of 3hr.

At about the 2hr mark the sparge tube plugged as evidenced by a lack of bubbles at the bubbler. This resulted in a small amount of suckback of
mineral oil into the reaction flask. Yes, a safety trap should always be used. The tube was removed and cleaned of a small amount of white solids.
After 3hrs the hydrogenation was stopped and the product poured into a 250mL beaker. From the beaker small test tubes were filled for use in a
centrifuge.

product before centrifugation
b. catalyst removal
The product was centrifuged using 4 small test tubes allowing processing at 30mL at a time. This nicely removed the catalyst providing a clear
product and a black residue. This clarity indicated that the fumaric acid was no longer in excess of saturation, hopefully having been converted to
the more soluble succinic acid.

centrifuged product
The clarified product was placed in an evaporating dish to air dry at room temperature. This took about a day. The presence of a small amount of
catalyst and mineral oil was evident.
Attachment: phps31yFS (128kB)
This file has been downloaded 1081 times
Clarified product in evaporating dish and the catalyst in water
The catalyst was washed into a small bottle with water and capped. This will be recovered for reuse if possible.
6. Recrystallization
The dried product crystals contained some ultrawhite crystalline contaminant as can be seen more clearly in the picture below. These formed in
clusters on the dish wall above the liquid level. That would have been the time to remove them. Instead I picked them out, placing them in a
separate beaker as shown below. I speculate that this is unreacted fumaric acid or a by-product.

dried product crystals sorted
The weight of the putative succinic acid was 6.33g; that of the ultrawhite crystals 0.59g.
The putative succinic acid crystals were recrystalized from 25mL of boiling water per Vogel. These were caught on a 7cm Buchner funnel as shown
below.

Recrystallized putative succinic acid
7. Results
The mp of the crystals was 235°C; the Vogel value is 184°C. Therefore the product is not succinic acid. Its composition is unknown.
8. Discussion & Conclusions
The Vogel procedure measures the volume of hydrogen consumed and specifies that the consumption will take about 2hrs. My apparatus did not allow
measurement of the hydrogen generated or consumed. Also, Vogel’s precursor was maleic acid which I assumed would hydrogenate easier than my
precursor, fumaric acid, due to steric factors. Therefore I hydrogenated for 3hrs as a safety factor.
Serendipitously the tramp mineral oil acted as an absorbent for the traces of Pd/C in my product. These were removed from the water surface using a
small cotton swab during the recrystallization.
Chemplayer has a YouTube up that shows how to make succinic acid from Na glutamate, available OTC as Accent seasoning/meat
tenderizer. This is likely a cheaper way to go considering the high cost of Pd/C even if maleic acid had been used and the synthesis had been
successful.
Apparently the steric factor, cis vs trans, 1,4-butenedioic acid, is very important here and the fumaric acid double bond failed to
take up the H2.
9. References
1. “A Textbook of Practical Organic Chemistry,” 3rd ed, (1956) by A. I. Vogel, pp. 473-474.
2. “Ullmann’s Encyclopedia of Industrial Chemistry,“ p. 8 under "Dicarboxylic Acids, Aliphatic."
3. “Introduction to Organic Laboratory Techniques,” (1998) by D. L. Pavia et al, pp. 121-126.
Your comments, suggestions, and recommendations are encouraged.
[Edited on 2-10-2016 by Magpie]
[Edited on 2-10-2016 by Magpie]
[Edited on 2-10-2016 by Magpie]
[Edited on 3-10-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
A couple of quick points. Its good to see you've used ethanol as solvent as its much safer around Pd/C than the commonly used methanol. Melting point
of your "product" is depressed with respect to fumaric acid, suggesting some conversion has taken place (especially as the observed melting point does
not correlate to any of the potential impurities you might expect to see). Ideally you'd do the hydrogenation under a balloon rather than continuous
sparging - you'll get better absorption and no flammable exhaust gas. De-gas reaction mixture with vacuum and back-fill with hydrogen (x3) before
heating. You can then just let it sit under balloon pressure. Make sure you have good stirring to facilitate gas transfer into solution. It may also
be possible that the 5% Pd/C is just not active enough, and 10% might yield a more favourable result.
You need to have some form of IPC so that you can determine any success without having to terminate the reaction; TLC would likely be the easiest to
employ if you have/can make plates. It might be that you'd see spot to spot conversion with a longer reaction period.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
I suspect that the product you isolated is mostly fumaric acid - how sharp was the mp? I also suspect that the reduction of fumaric acid requires some
positive H2 pressure because, as you suspected, sterics may play an adverse role.
Several alternatives are possible but may be no more successful:
1. Run the reaction in water on a salt of fumaric acid.
2. Use a transfer hydrogenation method so the entire reaction is "one pot."
It all depends on how badly you want succinct acid though I do realize that you are doing a demo experiment.
Finally, for safety reasons, methanol is one of the worst solvents for Pd/C hydrogenations. Flash fires are a constant worry using dry catalyst. If
methanol is the choice then a wet (H2O) catalyst should be used. 95% ethanol is a fairly standard solvent.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you both for the good comments.
I mentioned my prior use of methanol only as it was the specified solvent in the experiment I did in a school lab, found in Pavia. But thanks for the
information/warning.
I used absolute ethanol as that was the callout in Vogel. I assumed that any water present could cause the formation of a malic acid by-product.
I don't have a tank of H2 so how do I fill a balloon? My gas pressure was 1" of mineral oil (0.8" H2O). I could have increased this by simply
putting more mineral oil in my bubbler. The H2 generation method I used worked well for hydrogenating methyl oleate using 10%Pd/C at room
temperature, no degassing, 1"mineral oil gas pressure.
The melting point was not sharp, 232-235°C. I would have tested my product for unsaturation if I would have had any bromine water handy. Also I
could have used KMNO4 water but I didn't think of this at the time and now the product has been discarded.
Is there an easy way to test the "activity" of my 5%Pd/C?
[Edited on 3-10-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
To test the Pd/C, just put a speck of it on some filter paper and add a few drops of MeOH. If it catches fire, it is highly active. Often 5% will
not catch. but 10% will catch much more easily. If you use Pd/C, you could just add ammonium formate to generate H2 in situ, as AvBaeyer suggested.
It is a nice, stable, easy to use source of hydrogen. That is a nice way to do it, can often just reflux the reaction to make the H2 and react it at
the same time. Maleic acid is not too hard to come by, if you want to try it, let me know.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I did your test Dr Bob: no combustion.  I then passed some warm propane over the
filter paper - still no combustion. I then passed some warm propane over the
filter paper - still no combustion.
I'm going to call the vendor, ChemCenter of La Jolla, CA.
It is labeled as 5g of Palladium on Carbon, 5% (Reduced, Eggshell, wet).
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
cubalibre
Harmless

Posts: 11
Registered: 3-10-2016
Member Is Offline
Mood: H2 saturated
|
|
Using a centrifuge for catalyst recovery sure seems nice.
Thanks for sharing with us Magpie!
I've ran some ballon reactions in the past without access to a H2 tank either.
I produced it by adding Al to an 1l Erlenmeyer filled with some aqueous NaOH solution. The ballon was connected via a three-way valve, that way you
can degas the solution and setup with argon first a few times. The aluminium was added under a stream of inert to prevent intoducing O2 to the system.
The reaction needs to be cooled externally and everything should be sealed using PTFE or Parafilm.
Same goes for running the reaction.
To test your catalyst just add it to some formic acid. If it is active you should notice gas production.
 
[Edited on 3-10-2016 by cubalibre]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks cubalibre for the procedure on filling a balloon. I don't have any formic acid handy.
I got a hold of Bill at ChemCenter. He was very accommodating and is puzzled as this is the first complaint. He said my product was taken from a
3-yr old bottle of Johns-Manville (now Alfa-Aesar). He's going to refund my money and send me a gram of 10%Pd/C gratis.
edit: I wonder if he did not mean Johnson-Matthey instead of Johns-Manville.
As a nice alternative try a Moscow Mule! 
[Edited on 3-10-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thats a good result, Magpie. I assume you'll be attempting the reaction again once the catalyst has arrived?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't have anymore fumaric acid. I do have 2-3g of maleic acid. I might try with that. I didn't really intend to make a research project out of
this
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Just out of curiosity i don't suppose succinic anhydride subliminates in the same way that phthalic anhydride does, does it?
Cheers Fish
|
|
|
cubalibre
Harmless

Posts: 11
Registered: 3-10-2016
Member Is Offline
Mood: H2 saturated
|
|
Magpie, did you ever notice any eggy smell when producing the H2?
I've had Zn/HCl reactions smell of H2S (?) with allegedly 99%+ zinc, it's a long shot but could be the case here too.
Maybe you poisoned your poor catalyst instead of saturating it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
No, no smell of H2S.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Maleic acid should work fine. Let me know if you need more of any of those chems.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for the kind offer Dr Bob.
Vogel also says that fumaric acid should work, but again without references, or at least ones I can access. One reference here may be parroting the
other.
Now that I know that my catalyst was bad I really want to see if fumaric acid would work.
I did get my money back from ChemCenter. Now I'm waiting for the 10%Pd/C.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Fumaric and maleic acids have given nearly identical yields in other cases, such as CrSO4 or Raney Ni+hydrazine hydrate...that mossy zinc can reduce
Cr+3/+6 to chromous BTW.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Reaxys search:
Attachment: MAHtoSAH.pdf (244kB)
This file has been downloaded 844 times
Attachment: MAtoSA.pdf (561kB)
This file has been downloaded 1393 times
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Wow, REAXYS is awesome! Would it be possible for you to post the first article in the 2nd link?
Thank you Waffles.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
| Quote: |
Layered double hydroxides supported nano palladium: An efficient catalyst for the chemoselective hydrogenation of olefinic bonds
Chemoselective hydrogenation of olefinic double bonds in the presence of various functional groups using layered double hydroxides supported
nanopalladium (LDH-Pd0) catalyst is described. LDH-Pd0 was recovered quantitatively by simple filtration and reused several times with consistent
activity and
selectivity.
Lakshmi Kantam M, , Parsharamulu T, Manorama S.V.Inorganic and Physical Chemistry Division, Indian Institute of
Chemical Technology, Hyderabad 500007, India
|
Attachment: m2012.pdf (2.5MB)
This file has been downloaded 789 times
[Edited on 7-10-2016 by Waffles SS]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you. The LDH-Pd catalyst seems extremely efficient and not to difficult to make.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
cubalibre
Harmless

Posts: 11
Registered: 3-10-2016
Member Is Offline
Mood: H2 saturated
|
|
Not bad at all the LDH-Pd.
Even leeching seems to be less of a problem (they only tested it on one substrate though) compared to Pd/C, no coking either .
Any idea how they compare to polymer-carried cat?
Count me in if you prepare it Magpie!
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Looks like it would be nearly ideal for making zingerone, rheosmin, or other phenylbutanones.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Good writeup, Magpie! I regret that I didn't see it earlier.
I have been working full time lately in a carbohydrate research lab. Around the lab I have gained a reputation for being the 'hydrogenation guy'. I
primarily do hydrogenation flow chemistry (look up H-Cube, I have fixed one of these and completely taught myself everything I know about it) and I am
the go-to person with Parr hydrogenation, too. Might I give just a couple quick tips?
a) I find that the most frequent time that the catalyst 'dies' is as soon as it enters the flask with your starting material. It tends to burn up in
the air, even if there is no 'bang.' Its strength is diminished. I found that if you just squirt some argon in before you dump in the Pd/C, it will
work a lot better. I know you did a purge, but was it enough?
b) You need to take every measure to make sure there is absolutely no sulfur anywhere in or near your setup. Sulfur is crazy poisonous to palladium
catalysts. Run a column. Recrystallize. Do something to be sure of it.
c) I found that the best solvent system is plain old methanol. If that doesn't work, try methanol/ethyl acetate 7:3. It honestly doesn't even need to
be all that dry.
d) A shaking motion actually creates a better surface than a stirring motion.
e) If possible, try Raney Nickel. That works especially well for double bonds.
f) I know that catalysts are expensive, but I always use a 1:1 ratio of catalyst to material I'm hydrogenating. Try a smaller scale, first?
g) Hydrogenations can take a long time. Try running it for 3 hrs, and then take a TLC plate for each following hour and compare it to the Rf of your
starting materials and expected product, if possible.
Just a couple things I learned! Let me know your thoughts on them! 
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for your input! You clearly have the benefit of a lot of catalytic hydrogenation experience.
My first thought was: this can't be all that complicated. After all this is a widely used technology that's been around for a long time. Also there
is a student lab manual (Pavia) that has an experiment for hydrogenating methyl oleate to methyl stearate using the exact same technology that I used
for the succinic acid attempt. Then I looked in my lab notebook from 13 yrs ago to see what my yield was for the oleate: 1.3%, a terrible yield.
Industry making margarine must surely do much better.
I reported the results of my "activity" testing with methanol per Dr Bob, and with propane. Do you agree that this indicates that my 5%Pd/C has low
or no activity? If not, what test would you recommend?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
cubalibre
Harmless

Posts: 11
Registered: 3-10-2016
Member Is Offline
Mood: H2 saturated
|
|
Quote: Originally posted by ScienceHideout  | Good writeup, Magpie! I regret that I didn't see it earlier.
f) I know that catalysts are expensive, but I always use a 1:1 ratio of catalyst to material I'm hydrogenating. Try a smaller scale, first?
|
This sounds excessive even for academia  . Your supplier must love you. . Your supplier must love you.
|
|
|
| Pages:
1
2
3
4 |