rashid
Harmless

Posts: 2
Registered: 27-9-2006
Member Is Offline
Mood: No Mood
|
|
hydrazodicarbonamide
hydrazodicarbonamide is an itermediate for the synthesis of azodicarbonamide which is used as a blowing agent in PVC and as doughing agent in flour.
Tell me about the synthesis of hydrazodicarbonamide by a cheaper and easily handled process.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I suspect that a patent search will turn up the more recent, better methods. It's not the sort of material hobby chemists typically deal with.
I do know a specialist who could do this research for you. They are fairly inexpensive, only about US$ 120/hr plus any special expenses such as
retrieval of patents from some countries.
If you are interested in producing picric acid from aspirin, pool chemicals, and garden fertilizer, there are several threads on that subject.
Enjoy.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
A New Preparationof gem-bis(Difluoramino)alkanes via Direct Fluorination of Geminal Bisacetamides
RobertD. Chapman, MatthewC. Davis, and Richard Gilardi
Synthetic Communications vol. 33, no. 23, pp. 4173–4184, 2003
ABSTRACT
A fundamentally new preparation of internal and terminal gem-bis(difluoramino)alkanes has been demonstrated by the direct fluorination of
corresponding gem-bisacetamides, specifically, 1,1-bisacetamidocyclohexane and 1,1-bisacetamidopropane, leading to 1,1-bis(difluoramino)cyclohexane
and 1,1-bis(difluoramino)propane, respectively.
Excerp
............This behavior of coupling by the carbamoyl group of
acetamide is analogous to that reported for urea by Glemser and
Lu¨ demann,[23] forming hydrazodicarbonamide from fluorination (1:2
F2/N2) of urea in a temperature range of 30 C to r.t. This course of
fluorination of a primary amide is distinctly different from that of
secondary amides, which proceed simply to N-alkyl-N-fluoroamides
and alkyldifluoramines.[24]
Ref: 23-Anorg. Allg. Chem. 1956, 286, 168–173.
Uber die Einwirkung von Fluor auf Harnstoff
Von OSKAR GLEMSER u nd HORSTL ~DEMANN~
Anorg. Allg. Chem. 1956, 286, 168–173
http://rapidshare.de/files/34773981/fulltext.html
Inhaltsubersicht
Bei der Einwirkung yon Fluor auf Harnstoff entstehen bei -30" C Hydrazodicarbonamid H,N-CO-NH-NH-CO-NH,, HF, NH,, COF, und CO,. Bei Raumtemperatur ist
das Hauptprodukt Hydrazodicarbonamid neben HF, NH,, COF?, CO, und Biuret. Hydrazodicarbonamid gibt beim Verseifen mit halbkonzentrierter Schwefelsaure
Hydrazinsulfat.
Aus dem Fluorgenerator stammendes OF, bildet mit NH, als Nebenprodukt NO
und NO,.
[Edited on 28-9-2006 by solo]
Attachment: a new prepration of gem-bis diflouroamino alkanes .pdf (182kB)
This file has been downloaded 1666 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
rashid
Harmless

Posts: 2
Registered: 27-9-2006
Member Is Offline
Mood: No Mood
|
|
temporary
| Quote: | Originally posted by MehdiBorghei
Hi everyone , I'm searching about the process of oxidizing HYDRAZODICARBONAMIDE due to producing AZODICARBONAMIDE which
is the Blowing Agent for PVC and many other polymers. It would be kind of you if answering this question and guiding me to solve this URGENTproblem.
my email is arses2002@yahoo.com , please inform me if you could help me . |
i am also working on the synthesis of azodicarbonamide and i have prepared it.
Let you please tell me about the synthesis of hydrazodicarbonamide. i am attaching the file for conversion of HDC to ADC.
Waiting for your answer.
Attachment: 00698721dis.pdf (222kB)
This file has been downloaded 1422 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
hydrazodicarbonamide (aka biurea) H2N-CO-NH-NH-CO-NH2
<b>Biurea from urea & hydrazine</b>
Six grams (0.1 mole) of urea was heated with 2.5g (0.05 mole) of hydrazine hydrate in a closed tube at 130° for 1 hr. The residue was recrystallized
from hot water. The yield of biurea was about 20% based upon the amount of urea employed.
<b>Chlorourea from urea, zinc oxide, chlorine</b>
Sixty grams (1 mole) of urea was dissolved in a minimum amount of water. To the resulting solution was added 120 g. (1.5 moles) of zinc oxide.
Chlorine gas was then slowly bubbled into the stirred reaction mixture. The temperature was maintained at or very nearly 0° during the addition of
the chlorine. As chlorine was added the reaction mixture became momentarily clear. Then a white precipitate began to form and the reaction vessel
became filled with a thick mass of crystals. The addition of chlorine was then stopped, but the agitation was continued a while longer to allow the
dissolved chlorine to react with the unchanged urea. The white flake-like precipitate was then removed by filtration. 65% yield
<b>Biurea from chlorourea and ammonia</b>
The chlorinated urea product (20-35% available chlorine) obtained above was dissolved in approximately 25 volumes of water. The resulting solution
was slowly poured into an excess of concentrated aqueous ammonia. A vigorous evolution of gas occurred and the solution became yellow in color. On
cooling a white precipitate formed. The resulting solid was separated by filtration and was crystallized from hot water,. mp- 255° dec.; yield 16%
[Edited on 29-9-2006 by Axt]
Attachment: reaction of chlorinated urea products.pdf (280kB)
This file has been downloaded 1721 times
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Is there a special reason to use zinkoxide? It looks like the reaction just needs a base added to neutralize the formed hydrochloric acid?
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Yes, Cl2 will add directly to urea, producing HCl. I imagine the ZnO is used to buffer the solution near neutral.
ZnO is a common ceramic pigment, or the main ingredient in calamine lotion if you want to go to that much trouble.
Biurea if treated with nitrous acid yields carbamonyl azide, which on hydrolysis gives ammonium azide. Thats why I had that article.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
VEry interesting. This would be a great route to azide, completely sidestepping the requirement of hydrazine which is a pain to make. Pity the yield
of conversion from chlorourea+NH3 is only 16 percent. I wonder what the mechanism of the reaction is btw. Lot's of gas is evolved, is that HCl? Also,
the chlorourea is dissolved in a large amount of H2O, to which conc NH3 is added. I wonder how wise it is to have such a large amount of H2O, because
it won't help the preciptiation of the biurea. Do you have possibly solubility data on biurea? Maybe this is why the yield is low, because lots
remains in solution.
Also chlorourea seems like an interesting starting compound...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
The article suggests that chlorourea might not be the reactive species producing biurea after treatment with ammonia, so this may be the cause for the
low yield. The reaction of dichlorourea with ethylamine produces diethylbiurea in 60% yield but this may also be due to a lower solubility in water...
But yeah, this is a great route to azides, avoiding the toxic and rather difficult to produce hydrazine intermediate. In fact, azodicarbonamide and
biurea even seem to be used as a dough improver. They mention calcium oxide (in aquous solution?!) as a replacement for the zinkoxide used. Even
calcium carbonate seems usable at first glance...
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
Do you have possibly solubility data on biurea? Maybe this is why the yield is low, because lots remains in solution. |
Only slightly soluble, 0.24g/l @ 16°C; 0.4g/l @ 25°C
So thats not the problem. Its likely what nitro-genes pointed out, its not even known what "chlorinated urea" product is the reactant forming biurea.
Im not sure how hard it is to form & isolate the carbamonyl azide from biurea. I only have an old german reference to it, but have never seen it:
Thiele, J., and Stange, O., Ann , 283, 1 (1894).
Carbamonyl azide (H2N-CO-N3) itself is an explosive, that forms metal salts.
Attachment: biurea-dataheet.pdf (39kB)
This file has been downloaded 1422 times
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Now that I think of it, the main reason for the low yield may be the formation of hydrazine after all. Chlorourea under basic conditions is known to
produce hydrazine in high yields upon fast heating and produces the yellow/orange solutions just like the article mentions. Since no glue is present,
the formed hydrazine is quickly oxidized yielding N2 and water that might explain the vigerous evolution of gas they are talking about. Simply keeping
the reaction temperatures somewhat lower may favor the formation of biurea instead of hydrazine...
[Edited on 30-9-2006 by nitro-genes]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Axt posted on 28-9-2006
Reaction of Chlorinated Urea products |
The article Reaction of Chlorinated Urea products
cites this reference for the preparation as done by Chattaway
Journal of the Chemical Society 1909 Vol 95 / page 235
In his preceeding investigations Chattaway noted that the yield of Dichlorourea
(in an an unbuffered preparation) depends on the pH and speed of the preparation
to reduce hydrolysis and side reactions with Chloramines. My guess hypochlorite
further accelerates the decomposition of the product. ( Cl2 + H2O <=> HCl + HClO )
Only 25 % yield is claimed
Attached article _
The Action of Chlorine upon Urea Whereby a Dichloro Urea is Produced
F.D.Chattaway
Journal Proceedings of the Royal Society of London. Series A
Vol 81, Number 549 / December 22, 1908
Pages 381-388
____________________________________________
Gassing dry Chlorourea with NH3 , the expected result would be as follows :
Monochlorourea , H2N(CO)HNCl + NH3 -> H2N(CO)HNNH2.HCl , Semicarbazide Hydrochloride
http://www.chemicalland21.com/lifescience/phar/SEMICARBAZIDE...
Dichlorourea , ClNH(CO)HNCl + 2 NH3 -> HCl.H2NNH(CO)HNNH2.HCl , Carbohydrazide Hydrochloride
http://www.chemicalland21.com/specialtychem/perchem/CARBOHYD...
In aqueous solvation the result instead must be :
For Monochlorourea , 2 H2N(CO)HNCl + NH3 -> HCl + H2NCl + H2N(CO)HNNH(CO)NH2 , Biurea alias Hydrazoformamide
Deceptively simple there must be considerable subtle and complex parallel reactions.
As in the formation of Chlorourea , HClO must also be present. The poor yield 16 % must be
attributable to side reactions analogous to the preparation of hydrazine , the presence of chloramines
and the increasingly acidified solution as the reaction proceeds. Buffering this reaction is even
more important than in the formation of Chlorourea itself. Effervescence indicates decomposition
until equilibrium is established. Trying to form a mildly basic compound from an acidic precursor in
an acid enviornment , how smart is that ? Note that Chlorourea is soluble in Alcohol and ether.
I 'm wondering if this could be done better adding Chlorourea to an alcoholic solution of ammonia
instead, with inclusion of chaulk CaCO3 or NaHCO3 to immobilize the chlorine released
On the pH of chloramine formation _
" Monochloramine , Dichloramine , and Nitrogen Trichloride mix of species produced depends
on the ratio of chlorine to ammonia and the pH of the water. In the pH range of 7 to 8 with
a chlorine to ammonia weight ratio of 3 to 1 ( 1.5 mols Cl to 1 of NH3 ), monchloramine is
the principal product. At higher chlorine to ammonia ratios or at lower pH values ( 5 to 7 )
some dichloramine wil be formed , equal amounts at 5.5 . If pH drops below 5 some
Nitrogen Trichloride may be formed."
Note that Biurea is formed initially in excess ammonia solution pH ≈ 11.5 !
Related patent process _
WO/2003/035601 _ Method & Apparatus for Preparing Hydrazo-Dicarbanomide using Urea as Starting Material
http://www.wipo.int/ipdl/IPDL-IMAGES/PCT-PAGES/2003/182003/0...
or same here _ ( EP1446378 )
https://publications.european-patent-office.org/PublicationS...
_____________________________________________
| Quote: | Axt posted on 28-9-2006
Biurea if treated with nitrous acid yields carbamoyl azide, which on hydrolysis gives ammonium azide |
Preparation of Carbamoyl Azide it seems then would be
Biurea , H2N(CO)HNNH(CO)NH2 + NaONO Nitrite -> H2O + NaO(CO)NH2 Sodium Carbamate + H2N(CO)N3 Carbamoyl Azide alias Azidoformamide
Carbamoyl radical H2NCO- yields Carbamic acid as well as Carbamoyl Azide H2N(CO)N3 , when hydrolyzed
H2N(CO)N3 + H2O -> CO2 + NH4N3 , the ammonium compound is obtained.
This cited reference - Thiele, J., and Stange, O., Ann , 283, 1 (1894)
along with another more recent two here below , are given in
Dictionary of Inorganic Compounds - Page 422 , here _
http://books.google.com/books?id=YmBiXJ7qRtAC&pg=PA422&a...
or here _
http://books.google.com/books?id=YmBiXJ7qRtAC&pg=PA422&a...
J. Thiele, et al, Justus Liebigs Ann. Chem., 1894, 283, 37(synth)
R. Hofsommer, et al,Z. Elektrochem., 1949, 53, 383 (synth)
A. Heesing, et al, Chem. Ber.,1970, 103, 539 (synth)
These patents relate to compounds similar to that of US2855435 cited in the article
Reaction of Chlorinated Urea products in the opening remarks of this post
Poly-azidoformamide US patent 3526644
http://books.google.com/patents/pdf/NH_C_NS.pdf?id=ZI51AAAAE...
Poly-azidoformamide US patent 3547843
http://books.google.com/patents/pdf/FOAMED_POLYOLEFIN_COMPOS...
.
Attachment: Dichloro Urea.pdf (1004kB)
This file has been downloaded 1521 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Synthesis can be achieved following:
1°)the reaction between hydrazine and methylchloroformate:
2CH3-O-CO-Cl +NH2-NH2 --> CH3-O-CO-NH-NH-CO-O-CH3.2HCl
2°)Reaction of the above with an oxydant like quinone, HNO3 or CuCl2
CH3-O-CO-NH-NH-CO-O-CH3.2HCl -ox-> CH3-O-CO-N=N-CO-O-CH3 + H2O (or -2H)
3°)Reaction of the azo compound obtained in 2 with NH3 aq concentrated:
CH3-O-CO-N=N-CO-O-CH3 + 2NH4OH --> NH2-CO-N=N-CO-NH2 + 2CH3OH
The free dicarboxilic acid is unkown but salts like potassium obtained by hydrolysis of the dimethylester is known and explosive.
The azo compounds of that family are all potent explosive and must be distilled under reduced pressure.
[Edited on 20-2-2008 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
WO/2003/035601 _ Method & Apparatus for Preparing Hydrazo-Dicarbanomide using Urea as Starting Material
http://www.wipo.int/ipdl/IPDL-IMAGES/PCT-PAGES/2003/182003/0...
or same here _ ( EP1446378 )
https://publications.european-patent-office.org/PublicationS...
Additional reference to above
http://books.google.com/books?id=Fqj4U1kliDYC&pg=PA290&a...
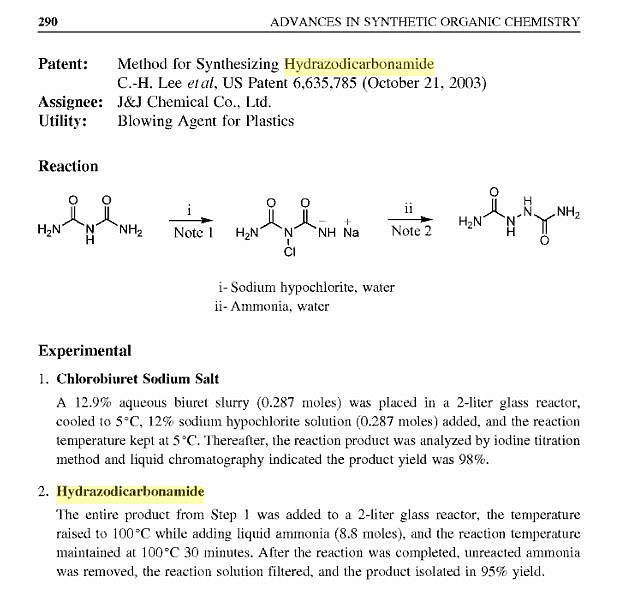
Method for synthesizing hydrazodicarbonamide
http://books.google.com/patents/pdf/Method_for_synthesizing_...
.
[Edited on 29-2-2008 by franklyn]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
hydrazodicarbonamide
Maybe this chlorourea route would be the easiest way, reported yield is 70%
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
Related patent is US3238226 Attached
http://www.google.com/patents?id=bnhKAAAAEBAJ&printsec=a...
If Azide is the intended end product, then attention is called to Example #20 of the patent shows a method for obtaining Semicarbazide as the product
in 90% yield. Byproduct hydrazodicarbonamide is separated by filtering and then byproduct hydrazine is separated by acidifying and filtering
from the more soluble Semicarbazide. This should not require isolation, but could be nitrosated to the Carbamyl Azide and then decomposed by heating
to leave Ammonium Azide in solution. If it was desired or necessary to isolate and purify the semicarbazide prior to nitrosation then acetone could
likely be used to form the semicarbazide addition product for isolation and that could later be converted back to semicarbazide.
Attachment: US3238226_SYNTHESIS_OF_HYDRAZINE_SEMICARBAZIDE_HYDRAZODICARBONAMIDE.pdf (344kB)
This file has been downloaded 1226 times
[Edited on 3-8-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Axt  | Yes, Cl2 will add directly to urea, producing HCl. I imagine the ZnO is used to buffer the solution near neutral.
ZnO is a common ceramic pigment, or the main ingredient in calamine lotion if you want to go to that much trouble.
Biurea if treated with nitrous acid yields carbamonyl azide, which on hydrolysis gives ammonium azide. Thats why I had that article.
|
Attached is an article which may be of interest with regards to the carbamyl azide, which may be obtained by reaction of nitrous acid and
semicarbazide.
Die Zersetzung des Carbaminsäureazids NH2CO.N3 für sich und in aromatischen Kohlenwasserstoffen
Theodor Curtius, Friedrich Schmidt
Journal für Praktische Chemie
1923, Volume 105, Issue 1, pages 177–198
DOI: 10.1002/prac.19231050115
Attachment: Die Zersetaung dos Carbarninslureadds NH,CO. N8 fir sioh nnd in aromatisohen Kohlenwasserstoffen.pdf (958kB)
This file has been downloaded 783 times
See thread on azides for additional references
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
Also attached
Ueber Semicarbazid
Johannes Thiele, Otto Stange
Justus Liebigs Annalen der Chemie
1894, Volume 283, Issue 1-2, pages 1–46
DOI: 10.1002/jlac.18942830102
Attachment: Ueber Semicarbazid.pdf (1.6MB)
This file has been downloaded 1040 times
Quote: Originally posted by Axt  | | Quote: | Originally posted by chemoleo
Do you have possibly solubility data on biurea? Maybe this is why the yield is low, because lots remains in solution. |
Only slightly soluble, 0.24g/l @ 16°C; 0.4g/l @ 25°C
So thats not the problem. Its likely what nitro-genes pointed out, its not even known what "chlorinated urea" product is the reactant forming biurea.
Im not sure how hard it is to form & isolate the carbamonyl azide from biurea. I only have an old german reference to it, but have never seen it:
Thiele, J., and Stange, O., Ann , 283, 1 (1894).
Carbamonyl azide (H2N-CO-N3) itself is an explosive, that forms metal salts. |
Also attached is
Carbamoyl Azides
Eugene Lieber, Ralph L. Minnis Jr., C. N. R. Rao
Chem. Rev.,
1965, 65 (3), pp 377–384
DOI: 10.1021/cr60235a003
Attachment: Carbamoyl Azides.pdf (709kB)
This file has been downloaded 980 times
[Edited on 10-8-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
2 methods for Biurea / Hydrazodicarbonamide
Related thread easier methods for Biurea / Hydrazodicarbonamide
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
US2692281 Biurea from hydrazine sulfate and urea
US3227753 Biurea or Hydrazine from Hypochlorite and Urea
Attachment: US2692281 Hydrazodicarbonamide from Hydrazine sulfate and Urea.pdf (76kB)
This file has been downloaded 843 times
Attachment: US3227753 Biurea (Hydrazodicarbonamide).pdf (254kB)
This file has been downloaded 846 times
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Do salts of hydrazodicarbonamide or azodicarbamide exist ?
like dinitrate?
|
|
|