Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
PID controller for electrolysis
Since some electrochemical reactions need a very stric temperature range (Molten NaOH electrolysis), and I am looking to do this, I thought, why not
use a PID controller to keep the temperature right?
To me, a PID works by switching the power on and off and thus achieving/maintaining a certain temperature, since NaOH electrolysis uses the
electrolysis current to keep it all molten you should be able to flash the power off when its too hot and on when too cold.
My only concern is that flashing on power in electrolysis is not very efficient, thoughts?
Thanks!
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
That is not exactly how a PID controller works. There are some controllers which mimic a PID controller with short burst of on and off control and
they can actually work pretty well.
A PID controller is called by that name because it reacts to whatever the variable it is in control with three mathematical thoughts, the P in the PID
controller is basically the proportion of response to the variable it is trying to control, the I implies the integral of the cycle of the variable it
is responding to, the D is the derivative or rate of change of the variable the PID controller is observing.
In your application the PID would probably get its info from the temp probe and then control the output from a variac or equivalent to control the
wattage to the heating element. Just know that PID controllers are not simple on off switches and the math used to tune them is not an intuitive
thing.
Some PID controllers come with auto tune, I have only see this option work properly a couple times over twenty years of a profession that dealt with
perhaps a couple thousand PID controllers.
One right out of the box may work for you, but if your system is dynamic I would suggest you do at least a bit of research.
For very many applications a simple P or proportional controller is the best and this can be achieved by simply reading the instructions from your PID
controller.
I am sorry for my babble sir but I have come across so many damned (so called PID gurus) who were so full of themselves (who had no clue on how to
marry the P to the I and the P and I to the D), these three are a team and I have seen them working together to calm some of the damndest dynamic
reactions.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
My cheap REX C-100 appeared to auto-tune quite well to a simple heater-thermocouple system, time constant I guess of around 1 minute.
Anyway, I doubt that fine long-term control is required here,
the c-100 appears to operate in burst mode ... on/off for varying intervals.
this allows relays to be used.
Mine is the zero-crossing ssr type, powered by the relay driver.
I would be most concerned with the cycling on/off of the power -
assuming an isolation transformer for low voltage;
the relay version will not last long due to contact arcing,
the ssr version like mine needs transient suppression.
Given that, I don't see why it should not work reasonably well with a REX C-100 or better.
If initial heating is electrical then a mechanical switch could select pre-heating or operate, with one thermocouple and controller.
EDIT: PID tuning is optional, you can choose just P by parameters.
manual
[Edited on 17-6-2016 by Sulaiman]
Attachment: rex-c100.pdf (362kB)
This file has been downloaded 657 times
Attachment: C100_400_700_900.pdf (843kB)
This file has been downloaded 532 times
I can't remember where the files are from, maybe SM even.
Plus, I have not used the ssr at high current yet, I don't beleive the rated 40A but maybe ok at max. 13A for UK, haven't tried yet.
I still can't believe the price http://www.ebay.co.uk/itm/220V-PID-REX-C100-Temperature-Cont...
[Edited on 17-6-2016 by Sulaiman]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
It's probably a better plan to operate the DC power supply at it's maximum capacity and use heating tape around the inner melt container to regulate
the temperature. I'm sure I've read about this working well.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by Marvin  | | It's probably a better plan to operate the DC power supply at it's maximum capacity and use heating tape around the inner melt container to regulate
the temperature. I'm sure I've read about this working well. |
I have also read this, but using power solely for heating would be a waste since more power in the bath means more things are getting electrolyzed!

|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Ultimately you have current doing useful work and I2R including waste heat just maintaining the melt temperature. I think what you are
saying is that running the cell with the wasted electrolysis power keeping the melt hot would be more efficient. You may or may not be right. You
are right that pulsing the power would waste energy.
Let's assume instead that the controller balances the steady current against the melt temperature. That's probably the lowest electrical power for a
given rate of sodium production... But only for that level of insulation. If more insulation is removed the cell might run at higher current and less
sodium metal might be lost through diffusion. Or the cell might be unstable through hydrogen explosions.
If you have a good high current power supply it makes sense to use as much current as possible or you waste the value. Twice the sodium produced at 3
times the cost could still be more value in a given unit of time even though the sodium costs more.
Most people just trying the experiment will probably be unable to keep the melt with the electrolysis supply they have and all the insulation they can
throw at it. They need a method of melting the solid hydroxide at the start of the experiment anyway.
Optimising the process would probably benefit from several years of experimental results and a chemical engineering degree and would also look at
electrode wear, cell life, cost of new parts and number of deaths per ton of sodium produced.
I'm starting to waffle, but my point is that it's easy to make a theoretical plan that ignores the big picture and often gets swamped by practical
problems. I succeeded in producing sodium on a small scale and failed scaling up to produce anything visible. I will probably try again knowing much
more now, but almost 2 decades on I'm not in love with going back to the fumes and sore eyes (behind goggles) bad electrical contacts and explosions
and loss of skin and everything corroding.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by Marvin  | Ultimately you have current doing useful work and I2R including waste heat just maintaining the melt temperature. I think what you are
saying is that running the cell with the wasted electrolysis power keeping the melt hot would be more efficient. You may or may not be right. You
are right that pulsing the power would waste energy.
Let's assume instead that the controller balances the steady current against the melt temperature. That's probably the lowest electrical power for a
given rate of sodium production... But only for that level of insulation. If more insulation is removed the cell might run at higher current and less
sodium metal might be lost through diffusion. Or the cell might be unstable through hydrogen explosions.
If you have a good high current power supply it makes sense to use as much current as possible or you waste the value. Twice the sodium produced at 3
times the cost could still be more value in a given unit of time even though the sodium costs more.
Most people just trying the experiment will probably be unable to keep the melt with the electrolysis supply they have and all the insulation they can
throw at it. They need a method of melting the solid hydroxide at the start of the experiment anyway.
Optimising the process would probably benefit from several years of experimental results and a chemical engineering degree and would also look at
electrode wear, cell life, cost of new parts and number of deaths per ton of sodium produced.
I'm starting to waffle, but my point is that it's easy to make a theoretical plan that ignores the big picture and often gets swamped by practical
problems. I succeeded in producing sodium on a small scale and failed scaling up to produce anything visible. I will probably try again knowing much
more now, but almost 2 decades on I'm not in love with going back to the fumes and sore eyes (behind goggles) bad electrical contacts and explosions
and loss of skin and everything corroding. |
thank you!
I suppose you would need something like a probe and an arduino to control the heating element/electrolysis current.
But thats all nice in theory, but I should just try it.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
just remembered :
SSRs leak current when off, enough for some people to feel
and never trust an SSR to protect you from mains voltages
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
New insights
Talking about reviving old threads....
Anyway, now that I finally got around to building my kiln and buying everything, I ofcourse thought about using a PID controller for some accurate
temperature control.
This controller would allow me to heat treat my knifes, cast metal at set temperatures and carbonize bread at a specific temperature 
However, this show opportunity too for some NaOH and NaCl electrolysis.
We know that Sodium dissolves into the melt at high temperatures, and the sweet spot to keep it molten and not dissolving is small, especially for
NaOH.
Thus, I devised a system, that would hopefully make it work.
I drew a rough sketch, Imgur link below.
http://imgur.com/a/4ZdmG
Quick explanation.
For starters, I used the art of weapons PID setup as a guide, he made a great video on his electric foundry by the way, would recommend. (https://www.youtube.com/watch?v=6fvBzlrlKl0)
My plan was to make a system that would turn off both the electrolysis current and heating coil when the melt got too hot.
Both the ATX PSU that will deliver the electrolysis current and the heating coil wire are controlled by the PID and SSR, I placed a voltage regulator
on the heating coil wire because 2000watts of raw power is a bit overkill for 300C NaOH, I will fine tune the voltage through experimentation so that
the heating coil plays a very minor role and keeping the current of the ATX unit running, instead of the power turning of constantly because the
heating coil was heating too much.
Both the ATX and the Heating wire will get a switch, along with a power indicator LED (Not pictured).
The thermocouple will go into the melt, so I can get the bath temperature accurately.
I do not mention cell construction here, this is purely the electrical system.
I know perfecting the process is extremely difficult, and dangerous, I do not expect perfection.
This project is a challenge for me, and ofcourse a learning experience.
The Cell/Furnace will also be used for melting metals and such, so I want to be able to use the Heating and PSU seperately, since I will not be using
a PSU while casting some alluminium.
Sorry for reviving such an old thread, but I thought this was not worth a new one.
I hope that some of you with more knowledge could review my design, and if there are flaws, point them out.
Thanks in advance.
Jstuyfzand
PS: Apologies for any grammar/spelling mistakes, should problably go to bed.
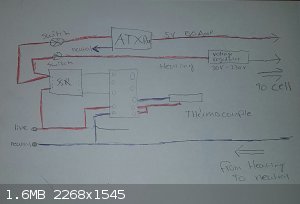
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
After some peer reviewing and trying a few things out, I realized that an ATX PSU does not like being shut on and off alot.
The green wire has to be connected with ground everytime the unit turns off, which would be a bit of a inconvenience, obviously.
I also presume that it is not good for the life of the unit, since those power supplys tend to be a bit more sensitive than a coil of nichrome.
I decided (But very much still open to critique) to exclude the PSU from the PID circuit, because I realize that 50A of current is not enough to keep
the bath molten, so it would be more effecient to let the electrolysis run continuously with the Coils only turning on when the bath temperature drops
too low.
PID and themocouple have been ordered, 2 actually, because they came from Hong Kong 
It was dirt cheap however, too bad the shipping is a few weeks at least.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I was about to comment on that, good for you figuring this out by your self. From a regulating POV it makes no sense switching more power than needed,
switching both heating and DC current will probably produce more fluctuations. The ideal setup would be to run a little less current than needed to
maintain the ideal temperature and regulate the rest with a heating coil. Don't push too much power through the coil however, the goal is to regulate
a slight deficiency with a slight excess.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by Fulmen  | | I was about to comment on that, good for you figuring this out by your self. From a regulating POV it makes no sense switching more power than needed,
switching both heating and DC current will probably produce more fluctuations. The ideal setup would be to run a little less current than needed to
maintain the ideal temperature and regulate the rest with a heating coil. Don't push too much power through the coil however, the goal is to regulate
a slight deficiency with a slight excess. |
Well said!
Unfortunately, I cant do any calculations on heat loss and insulations since my furnace is a bit crude, and the electrolysis current will fluctiate
through operation.
So I can not exactly know how many watts I need to keep it at the perfect temperature, I hope to find a sweetspot through practise that the heating
coil will stay on continuously at a lower voltage to compensate for the cooling of the bath since the electrolysis current wont be enough.
I suppose that running it continuously at a lower voltage/current is better than switching it on and off and making it cycle through temperature
differences of ~1000C.
Also, since you did not comment on any hypothetical mistakes in my circuit, I presume no obvious fatal errors are present?
Apart from the ATX psu being switched by the SSR ofcourse.
The PID controller I'll be using is a REX C100, not sure if that matters but I guess it will not do harm to mention it.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
It's a bit hard to read the schematics, but you seem to understand the general principles.
For proper regulating some swithching will be needed. That's OK, with a SSR you can reduce cycle time to one or two seconds (IIRC 2 is recommended).
It's basically a slow pulse-width modulation giving very precise control.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by Fulmen  | It's a bit hard to read the schematics, but you seem to understand the general principles.
For proper regulating some swithching will be needed. That's OK, with a SSR you can reduce cycle time to one or two seconds (IIRC 2 is recommended).
It's basically a slow pulse-width modulation giving very precise control. |
The parts are on their way, so the only way to find out if it would work is to make it, breaking the components should be hard, so I can just
rearrange it if stuff doesn't work.
Soon we will be able to throw sodium into wate... erm... dry organic solvents.
Len1 made an incredible cell, but looking on youtube I saw another design which looked very good:
https://youtu.be/BElkJ6VNwO4
If anyone reading would like to attempt NaOH electrolysis, this video gives a nice amateur setup instead of the really shitty ones, or the industrial
ones. A nice middle ground.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jstuyfzand  |
Len1 made an incredible cell, but looking on youtube I saw another design which looked very good:
https://youtu.be/BElkJ6VNwO4
If anyone reading would like to attempt NaOH electrolysis, this video gives a nice amateur setup instead of the really shitty ones, or the industrial
ones. A nice middle ground. |
I watched that video, his container is made of aluminium as is the seperator ??? The container may be covered with fire cement but the seperator is
not and where is the central electrode. I must be missing something.
Aluminium in molten NaOH. Can anyone explain this to me?
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by wg48  | Quote: Originally posted by Jstuyfzand  |
Len1 made an incredible cell, but looking on youtube I saw another design which looked very good:
https://youtu.be/BElkJ6VNwO4
If anyone reading would like to attempt NaOH electrolysis, this video gives a nice amateur setup instead of the really shitty ones, or the industrial
ones. A nice middle ground. |
I watched that video, his container is made of aluminium as is the seperator ??? The container may be covered with fire cement but the seperator is
not and where is the central electrode. I must be missing something.
Aluminium in molten NaOH. Can anyone explain this to me?
|
The reaction of NaOH and Al to form sodium aluminate requires the NaOH to be aquous, I think, since this is the reaction:
2 Al + 2 NaOH + 2 H2O → 2 NaAlO2 + 3 H2
In the comments he mentioned only having corrosion issues when he was washing the cell, and thus adding water to the mix.
Alluminium as a cell body could be very convenient, since you can cast Al relatively easily, you just need to be careful with water.
Although, in my experience solidified NaOH that has been molten barely dissolves in water, calcination or thorougj dehydration of the molecule maybe?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If sodium orthoaluminate (Na3AlO3) is formed, then the reaction is possible:
2 Al + 6 NaOH = 2 Na3AlO3 + 3 H2
I wouldn't trust an aluminum component in contact with molten NaOH.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Thats for sure, what if you would store it and some moisture got into the bath? A leaky container letting molten NaOH flow through your furnace when
you dont notice the holes.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Apparently the reaction of the aluminium with the NaOH is not very rapid. Perhaps its limited by insoluble products of the reaction or see below.
The narration says the cathode is black nickel wire surrounding the outside of the separator which would mean the sodium is formed on that electrode
and not inside the seperator.
Its difficult to see but judging from the neg sign on the caps and the wiring, the cathode is the aluminium which makes more sense as no electrode is
seen inside the separator. That would also tend to stop the aluminium dissolving in the NaOH. But it also means oxygen from the anode wire will mix
with hydrogen and most of the sodium in the space above the NaOH in the outer part of the chamber. Perhaps that why he had explosions.
Not a very well thought out set up with inaccuracies and not worth repeating.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by wg48  |
Apparently the reaction of the aluminium with the NaOH is not very rapid. Perhaps its limited by insoluble products of the reaction or see below.
The narration says the cathode is black nickel wire surrounding the outside of the separator which would mean the sodium is formed on that electrode
and not inside the seperator.
Its difficult to see but judging from the neg sign on the caps and the wiring, the cathode is the aluminium which makes more sense as no electrode is
seen inside the separator. That would also tend to stop the aluminium dissolving in the NaOH. But it also means oxygen from the anode wire will mix
with hydrogen and most of the sodium in the space above the NaOH in the outer part of the chamber. Perhaps that why he had explosions.
Not a very well thought out set up with inaccuracies and not worth repeating.
|
He mentions a nickel anofe situated in the bath, going into the collection tube. Thats also the reason for the position guide bolts, so that the anode
fits into the collection tube properly.
His design resembles the wikipedia picture of a castner cell, am on mobile so I can't link it.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jstuyfzand  |
He mentions a nickel anofe situated in the bath, going into the collection tube. Thats also the reason for the position guide bolts, so that the anode
fits into the collection tube properly.
His design resembles the wikipedia picture of a castner cell, am on mobile so I can't link it. |
The sodium usually forms on the cathode.
Perhaps the connection that goes to the bottom of the cell is a connection to a cathode that sticks up into the middle of the seperator and insulated
from the alluminium. That would account for why significant sodium is in the middle of the seperator. I did wounder how it got there if the seperator
was the cathode.
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by wg48  | Quote: Originally posted by Jstuyfzand  |
He mentions a nickel anofe situated in the bath, going into the collection tube. Thats also the reason for the position guide bolts, so that the anode
fits into the collection tube properly.
His design resembles the wikipedia picture of a castner cell, am on mobile so I can't link it. |
The sodium usually forms on the cathode.
Perhaps the connection that goes to the bottom of the cell is a connection to a cathode that sticks up into the middle of the seperator and insulated
from the alluminium. That would account for why significant sodium is in the middle of the seperator. I did wounder how it got there if the seperator
was the cathode. |
Exactly that, he must have mixed up the polarities of the electrodes.
And yes, Sodium forms on the cathode, brain fart 
[Edited on 11-12-2016 by Jstuyfzand]
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:06 |