| Pages:
1
2 |
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Chemicals from plants
I have been looking up information about some chemicals that can be extracted for plants (Probably not in a big amount, but as a just-because its a
plant, and its cool because nature). This came from some capsacinoids I extracted (in low purity though, I didnt have good ventilation or safety of
any way, as I was doing it with high alcohol conc vodka, and it started to feel quite unconfortable to my lungs and nostrills)
I ended up with some really spicy sauce, that even wetting a spoon and moving it arround some bacon, made that bacon way too spicy. I planted some
peppers, including carolina reapers, and I plant to repeat this when they grow, with the carolina reapers.
So this got me wondering, what other chemicals of "interest" could be extracted from plants?
I know toluene was named after the balsam of tolu, if im not mistaken a esential oil from a tree autoctonous in south america. If I had access to
that plant, I would be trying it for sure, but its a tree, so planting it to harvest it would require too much time.
Another idea I had, is extracting formic acid and histamine from nettles, to watch the world burn. However it is kinda scary. I can harvest tons of
nettles, since they grow a lot in the countryside.
Cianyde from apples, bitter almonds (Arround 50mg/almond acording to what I found online) However, again, kinda scary to play arround with cyanide for
me without a formation.
Menthol from mint.
Alliicin from garlic.
I would like to know how viable it is to purify these (having a pretty crude product at the end still) and any other ideas of interesting compounds
that could be stracted from plants.
[Edited on 9-6-2016 by ficolas]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I am also into plant extractions although i am very new to it. I have recently extracted as yet unknown 'things' from cloves.
I am working on extracting menthol from mint. There is a old book on the net ironically called a modern herbal. It gives you a good idea what
different plants contain.
I saved some papers from the mint and will post them when i find where i saved them!
This one was from eugenol i was trying to extract from cloves, there are some pics in the beginners section that i posted.
[Edited on 9-6-2016 by NEMO-Chemistry]
Attachment: eugenol.pdf (144kB)
This file has been downloaded 1002 times
And the menthol one from ment and how to get the menthol crystals, i havnt completed this one yet.
[Edited on 9-6-2016 by NEMO-Chemistry]
[Edited on 9-6-2016 by NEMO-Chemistry]
[Edited on 9-6-2016 by NEMO-Chemistry]
[Edited on 9-6-2016 by NEMO-Chemistry]
Attachment: menthol.pdf (120kB)
This file has been downloaded 793 times
|
|
|
Scalebar
Hazard to Self
 
Posts: 54
Registered: 6-5-2016
Location: Europe!
Member Is Offline
Mood: Looking for a way out
|
|
Here's a link to Grieves herbal http://www.botanical.com/botanical/mgmh/mgmh.html
Even elements like iodine used to be extracted from plant material - sea weed and high quality silica from horsetails.
Formic acid would be an easy target as it can be distilled - used to be extracted from ants (good luck getting them into the flask)
Caffeine is a fun one to try - the chemistry is simple but it still takes a degree of care and attention
|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Ants... killing ants while extracting formic acid... Sounds fun huehuehue
Is it ethical to put them inside the solvent alive? Or to boil them alive?
Ok just kidding, but there is an ant plague arround, so I may try this. However extracting it from nettles seems more ethical and less disgusting
There is another thread (http://www.sciencemadness.org/talk/viewthread.php?tid=66475) that was posted simoltaneously to this one, lets better use the other one, since it
was posted first
[Edited on 9-6-2016 by ficolas]
|
|
|
Scalebar
Hazard to Self
 
Posts: 54
Registered: 6-5-2016
Location: Europe!
Member Is Offline
Mood: Looking for a way out
|
|
I've never done it but I've seen it in ancient text books - it's a dry distillation, i can only assume that the ants were killed first as red ants get
quite angry when fiddled with.
I make Birch tar from time to time but it's hardly extracting a pure chemical.
Some of the more exotic henbanes contain enough alkaloid that it forms ( microscopic) crystals in the dry leaves.
One i really fancy doing is something that bridges the chemical/life divide - extract enough tobacco mosaic virus to form a visible crystal but I'd
basically have to kiss goodbye to every solanaceous plant in the garden and the allotment association would kill me.
|
|
|
Chem Rage
Harmless

Posts: 28
Registered: 28-3-2015
Member Is Offline
Mood: No Mood
|
|
I am quite into Natural Product chemistry, too. I grow all manner of different plants for their essential oils and the constituent chemical components
(e.g. terpenes) of these oils. A Soxhlet extraction apparatus comes in handy.
The Myrtaceae family, which includes eucalypts, have interesting phytochemicals.
http://www.pranarom.com/en/our-products/essential-oils/essen...
[Edited on 10-6-2016 by Chem Rage]
[Edited on 10-6-2016 by Chem Rage]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'd assume you could extract oxalic acid from rhubard or skunk cabbage. Never tired, though.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Chem Rage
Harmless

Posts: 28
Registered: 28-3-2015
Member Is Offline
Mood: No Mood
|
|
Indeed you could; it is a matter of harvesting enough raw material! Judging by the way things are going here in the UK (we are subjected to
increasingly draconian EU restrictions and bans), one would have to resort to rhubarb for their oxalic acid. I can only hope that Britain leaving the
EU nullifies these restrictions so that hobbyists here in Britain can once again enjoy good access to chemicals.
[Edited on 10-6-2016 by Chem Rage]
|
|
|
Cucurbit
Harmless

Posts: 17
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
Interesting ideas!
I once made a bit of benzoic acid from benzoin resin (styrax), cool.
Hmm, what else springs to mind...
- salicylic acid from willow bark
- limonene from orange peels (steam distillation)
|
|
|
Chem Rage
Harmless

Posts: 28
Registered: 28-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Cucurbit  | Interesting ideas!
I once made a bit of benzoic acid from benzoin resin (styrax), cool.
Hmm, what else springs to mind...
- salicylic acid from willow bark
- limonene from orange peels (steam distillation) |
Methyl salicylate from wintergreen
Piperidine from black pepper
Bergamottin from bergamot orange (citrus bergamia)
1,8 cineole, etc, from eucalyptus
Myristicin from nutmeg
Cinnamaldehyde from cinnamon
Citronellol and citronellal from lemongrass
Crocetin and safranal from saffron crocus
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2789
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Coniine is a useful one, found in pretty good quantities in a very common plant -- poison hemlock.
http://en.wikipedia.org/wiki/Coniine
There are a handful of procedures in chemistry that call for "a secondary amine" to act as a catalyst, such as the synthesis of MVK from acetone +
formaldehyde + diethylamine HCl; coniine may substitute for the inaccessible diethylamine.
http://www.orgsyn.org/demo.aspx?prep=CV4P0281
https://en.wikipedia.org/wiki/Methyl_vinyl_ketone#Production
Coniine may also react with organolithiums to produce lithium coniide, which is similar to but not quite as hindered as lithium diisopropylamide.
Diisopropylamine is easier to make of course (isopropyl iodide + NH3) than diethylamine.
Advantages over piperine/piperidine: can be extracted by A/B methods, does not require hydrolysis.
Disadvantage: coniine is very toxic, but not too much moreso than some of the more caustic things we deal with. It just has to be treated with respect
to the fact that it's more dangerous than it looks.
Mandelonitrile and benzaldehyde from bitter almond oil are also pretty useful, although I don't know if anyone has found a safe way to extract
mandelonitrile reliably. Cyanide is nothing to trifle with.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
The other thing that would be handy is a way to test that we have correct product, i intend to use TLC to see what compounds have been extracted, i
assume i need to put several dot on the plates, then test the bands with various reagents to see which which are alkaloids etc?
Slightly disjointed having two thread but choose one and i will post what i find in it, for reference the other thread is here http://www.sciencemadness.org/talk/viewthread.php?tid=66475
It would be easier to post papers etc in one place however.
I am particularly looking extraction that produce crystals, it would be nice to have a slightly different crystal collection.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
This seems pretty good, it details different methods and gives some plant families and the main oils obtained.
Attachment: Aromatic plant extr methods.pdf (2.2MB)
This file has been downloaded 794 times
Seems parsley is another source of myristicin, although at 38mg per Kg its hardly a huge source  , but posted anyway in case its useful. , but posted anyway in case its useful.
[Edited on 11-6-2016 by NEMO-Chemistry]
Attachment: parsely.pdf (799kB)
This file has been downloaded 676 times
|
|
|
BILLBUILDS
Hazard to Self
 
Posts: 82
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
does anyone know how to make/distill birch oil/resin
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Birch oil is methyl salicilate, right? Same as oil of wintergreen?
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
You wouldn't have to kill the ants if one can find a wood ant nest (they sometimes form huge piles, favouring pinaceous tree needles, so look for them
in acidic forests dominated by pines, larch etc (if your lucky there might be larch bolete and slippery jack mushrooms too, a type of bolete (Suillus
to be precise) which are both common, in season and very good just fried in butter after removal of the slime surface layer which peels easily). Note
certain folk are intolerant to the slippery jack, like some are to honey fungi or sulfur polypore, although for most both of these too are excellent
edibles. And there is always the possibility of saffron milk-caps, Lactarius deliciosus (along with the very similar, also edible but not as tasty
L.deterrimus, which stains with age more green)
Wood ants will spray formic acid when provoked into annoyance. Birds, especially the intelligent corvidae use this to rid themselves of parasites, by
pissing off a wood-ant nest and then standing back to be hosed down in acid.
Also birch produces two other interesting compounds, betulin and betulinic acid. with anti-cancer properties, betulinic acid being the more
bioavailable, and synergistic against certain cancer types, particularly those stemming from neurological progenitors. IIRC its (betulin the less
bioavailable, but naturally available given the profusion in the northern hemisphere of silver birch) also a GABAa positive allosteric modulator,
benzodiazepine binding site IIRC.
http://cat.inist.fr/?aModele=afficheN&cpsidt=20572488
|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
I've read about octyl acetate being present in citrus fruits (there are also oils that smell of citrus that likely contain a higher concentration).
However, I've haven't tried it myself as of yet.
http://www.hmdb.ca/metabolites/HMDB38602
https://en.wikipedia.org/wiki/Octyl_acetate
https://pubchem.ncbi.nlm.nih.gov/compound/Octyl_acetate
http://www.sciencemadness.org/smwiki/index.php/Octyl_acetate
Osage oranges (which I think are generally exclusive to the southeast U.S) supposedly contain a flavonol called Morin.
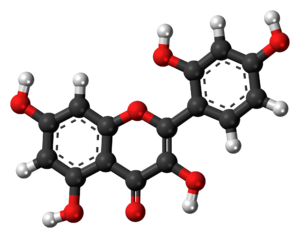
I've seen these oranges around where I live. I think I may try to extract it.
Apparently it creates fluorescent complexes with tin and aluminium in solution which would be a good indicator of a successful extraction.
Osage Orange: https://en.wikipedia.org/wiki/Maclura_pomifera#Distribution
Morin: https://en.wikipedia.org/wiki/Morin_(flavonol)
Morin MSDS : http://www.sciencelab.com/msds.php?msdsId=9926149
Current Active works in Progress:
-Convert i-propanol to i-chloropropane using HCl and ZnCl2
-Determine properties and solubility of a glycerol and citric acid polymer
-Attempt a conversion of chloroform to dichloromethane
-Attempt a conversion of acetic acid to acetamide
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
They use them in the Northeast as hedges, and they can occasionally be found growing wild even in pretty cold climates. The fruits look and smell like
green oranges but have a milky latex sap and are seemingly inedible.
|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
Quote: Originally posted by JJay  | | They use them in the Northeast as hedges, and they can occasionally be found growing wild even in pretty cold climates. The fruits look and smell like
green oranges but have a milky latex sap and are seemingly inedible. |
I had gone to a camp and asked about their edibility. The camp leaders said they were poisonous but people in older days had cooked Osage oranges and
were then able to eat them after the poisonous substances were leeched out.
In contrast, these sites say they're edible but the seeds are poisonous only...
http://www.eattheweeds.com/maclura-pomifera-the-edible-inedi...
http://www.wildflower.org/expert/show.php?id=5764
https://hortnews.extension.iastate.edu/1997/10-10-1997/hedge...
http://web.extension.illinois.edu/dmp/palette/061022.html
Some sites support it in use of home-made insect repellents or home remedies.
Most sites also seem to focus on the edible and 'positive' parts of it but I couldn't find much information on what substances could be the toxins in
the seeds of the orange.
I assume that if I were to attempt an extraction of Morin, I could likely just remove the seeds the first then attempt an extraction?
Current Active works in Progress:
-Convert i-propanol to i-chloropropane using HCl and ZnCl2
-Determine properties and solubility of a glycerol and citric acid polymer
-Attempt a conversion of chloroform to dichloromethane
-Attempt a conversion of acetic acid to acetamide
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Generally one should avoid eating or drinking from plants with milky sap, as a general rule of thumb. Most are toxic. The barrel cacti are one of the
main exceptions, but its vital to make sure that they are not in fact, Euphorbia or within that family. It packs some really pretty noxious, corrosive
phorbol esters in many of them, and some species can be lethal. Also the sap of many or most Euphorbia can blind if it gets into the eyes. And many
of them look a lot like true cacti.
Valeriana officianalis contains some intriguing compounds, GABAa positive allosteric modulators, and compounds close in structure to valproate
(epilim, the anticonvulsant). Mild to moderate sedative properties. The interesting bit is that at least one of its constituents, not sure which,
binds to the loreclezole-sensitive allosteric site on GABAa as a positive allosteric modulator. I find that taking a high dose of root extract is the
most potent and effective oneirogen I've ever encountered. Would love to bioassay loreclezole itself to see if this is the cause since I have never
ingested anything else that is an agonist of this binding site on GABAaRs. Just be prepared for a bit of a kicking in terms of intensity.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
That is true.Octyl acetate has citrus fruits odor and is very similar to orange fruit smell.(I have made it from 2-Octanol and 1-Octanol)
One of interesting natural extraction that i did is Stevia sugar(steviol glycosides) from Stevia plant.
Separation steviol glycosides from poly phenols(cause bitter taste) is challenge able and need special technique.
It is 200-300 times sweeter than table sugar
https://en.wikipedia.org/wiki/Stevia
[Edited on 18-3-2017 by Waffles SS]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Figs and dandelions are also exceptions to the "milky juice = bad" rule, but it's still a good starting point.
Extracting dyes form plants is a fairly common starting point- not least because you can tell where the stuff is when you do extractions etc.
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Yes, there are exceptions, I was just pointing out that GENERALLY its an indicator that the plant has likely noxious character. Some of the
Euphorbiales are not too bad but there are some exceptionally virulent species also. Manchineel (Hippomane mancinella) for example will apparently
blister and strip the paint off cars, just from the exudates washed during rain off the leaves. And caribbean tribespeople would use the sap as an
arrow poison, and even being near the tree will blister and burn flesh. I've read of people using it to kill people by means of tying them to the tree
trunk and leaving them there, to be slowly flayed alive as a method of execution/murder.
|
|
|
Booze
Hazard to Others
  
Posts: 121
Registered: 26-2-2017
Member Is Offline
Mood: No Mood
|
|
Well, for extracting capsacinoids I used 95% ethanol I made. It made a really thick, red oil that was really spicy, even an amount I couldn't see with
my naked eye burned for a few hours.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
As someone who's done a LOT of extractions, I recommend learning the difference between:
Tinctures
Concretes
Absolutes
Attars
and Essential Oils.
The right route, using the right solvent, is what will often determine if you get what you want.
|
|
|
| Pages:
1
2 |