Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
preparation of congo red
Introduction
Congo Red is an azo dye formed from the coupling of benzidine HCl and Na napthionate.
The benzidine HCl must first be diazotized as shown in this equation:
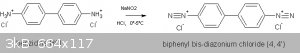
The diazonium compound is then coupled with the Na napthionate as shown here:
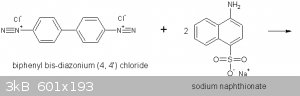
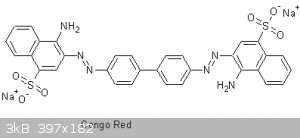
Congo Red is a direct dye (requires no mordanting) for cotton. It can also be used as an acid-base indicator turning from blue to red in the pH range
3.0-5.2.
Experimental
The reagents required for this preparation are shown in the photo below:

reagents required to make Congo Red
The procedure followed is that of reference 1.
The basis of my synthesis was 3.5g of napthionic acid. This was converted to Na napthionate by dissolving it in a minimum amount of 5% NaOH, ie,
~30mL.
Next the benzidine was converted to benzidine HCl by dissolving it in 9mL of boiling water to which had been added 1mL of con HCl.
a. diazotization
The benzidine HCl solution was then cooled to between 0°- 5°C in ice-water and some small pieces of ice added. 0.6mL of con HCl was then added
followed by a solution of 0.29g of NaNO2 dissolved in 6mL of water. The NaNO2 solution was added a few drops at a time until an excess was detected
using starch-iodide paper (all of it was required). This completed the diazotization.
b. coupling
A picture of the two reagents just prior to coupling is shown below:

Na napthionate (left) and diazotized benzidine HCl (right)
The diazonium solution was then run into the Na napthionate solution while stirring. The red color of congo red was immediately noticed as the
solution was already apparently at a basic pH.. Stirring was continued for an additional half hour.
c. Na2CO3 addition
Per procedure 0.8g of Na2CO3 was added which resulted in foaming. The stirred mixture was then heated to 80°C and maintained there until all
evolution of gas had ceased. The picture below shows the Congo Red, and 1 drop placed in water.

Congo Red solution and 1 drop in water
d. drying
The Congo Red solution was then placed in a 6” evaporating dish and taken to dryness on a steam bath as shown below:

Congo Red on steam bath
e. extraction with 90%ethanol
The dried Congo Red was then taken up with 6 x 37mL portions of boiling 90% ethanol. A picture of the last extraction is shown below:

Last extraction with 90% ethanol
Which left the residue shown below:

Residue following the extraction with 90% ethanol
This residue is composed of organic material and NaCl. During disposal I noticed that it was nearly all soluble in water.
The ethanol extract of Congo Red is shown below:
Attachment: phpquj4qR (117kB)
This file has been downloaded 922 times
Ethanol extract of Congo Red
f. removal of ethanol by distillation
A little more than half of the ethanol was then removed by distillation. The Congo Red pot residue was then placed in an evaporating dish. This is
being taken to dryness by evaporation.
g. purification
I feel that my product is still has significant contamination, due to its dark color and due to information presented in reference 2. Once my ethanol
extract has dried I intend to purify the Congo Red according to the method presented in reference 2.
Results
Final results are pending the planned purification. Following this I will be able to determine the yield and compute a % yield. I should also be
able to post a picture of an improved product (a powder).
Discussion
As shown above the color of the sodium napthionate solution and the diazonium solution are dark. I don’t have an explanation for this darkness
other than possible contamination due to tar.
Reference 1 indicates that the product would change to a red color once the Na2CO3 was added. My product was already red. I don’t understand this
discrepancy.
The Kline procedure specifies 6g of napthionic acid for every 1g of benzidine HCl. This is a mole ratio of 6.9! The stoichiometric mole ratio is
only 2.0. Why this huge discrepancy?
References
1. “CONGO RED,” Journal of Chemical Education, by E. R. Kline, (Mar 1938), pp. 129-131.
2. “A METHOD FOR THE PURIFICATION OF CERTAIN AZO DYES,” Industrial and Engineering Chemistry, by H. E. Lubs, (Feb 1919), p. 456.
Questions, comments, and suggestions are welcomed.
[Edited on 31-5-2016 by Magpie]
[Edited on 1-6-2016 by Magpie]
[Edited on 1-6-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Beautiful - first word that came to mind.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Did you purify it further yet? Nice work.
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have not read through this thoroughly yet. (life=chaotic atm.) But this looks like a lovely bit of work and well executed. This is what this
board is about. Kudos.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks to all. I'm still working on the purification. The ethanol extract has just reached dryness in air.
The question I ask in the Discussion about the high mole ratio really bothers me. The procedure in Vogel has a much more reasonable mole ratio, ie,
2.4 IIRC. I would hate to think that I wasted all my hard-won naphthionic acid due to a mistake in the Kline procedure.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is an update on my attempted purification of homemade congo red. The method I am using is that by HA Lubs (1919). The intent of the procedure
is to yield pharmaceutical grade congo red, ie, essentially pure congo red.
This purification is not going well in my hands. The basis of the procedure is to saturate an aqueous solution (colloidal suspension) of congo red
with sodium acetate, resulting in precipitation of the congo red. This entails adding a huge amount of sodium acetate.
My dried ethanol extract weighed 5.48g. This is way too high as the theoretical maximum is 1.65g. So, obviously purification was in order.
Following the Lub procedure I dissolved the product in water (~40mL) then filtered it removing considerable solids which were discarded.
Again, following procedure I added 35g of sodium acetate. This is where things went south. I'm having trouble separating out the congo red. The
picture below shows all products since the original filtration minus the solids discarded.
The dark solid on the 7cm filter paper is the small amount of congo red. I'm tempted to just stop at this point before I do any more damage. If I
make a 0.1% ethanol solution with this I will have an adequate amount of indicator for my needs.
What I really need at this point is a solvent that will take up congo red but not sodium acetate. I feel that this is quite a challenge as what I
have is two sodium salts of organic acids, and their solvents/non-solvents will be identical.
Any suggestions will be greatly appreciated.

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unionised
International Hazard
    
Posts: 5134
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
I feel that this is quite a challenge as what I have is two sodium salts of organic acids, and their solvents/non-solvents will be identical.
|
The sulphonic acid is much stronger than the carboxylic acid.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Fundamental Processes of Dye Chemistry has a preparation which might be of some use. | Quote: | A solution, prepared by tetrazotizing 18.4 grams of technical benzidine
(page 261), is mixed with a solution containing 50 grams of naphthionate
and 50 grams of sodium acetate in 200 cc. water. The temperature
is held at 5°C. for 1 hour, then raised slowly to 20° where it
is held for 5 hours. Subsequently, the mixture is stirred at 30° for 24
hours, and then, on the third day, is heated to 55°. After the coupling
has proceeded for 2.5 days, the reaction mixture is heated to boiling
and treated with 40 grams of calcined magnesia, whereupon the insoluble
magnesium salt of Congo red is precipitated. The salt is filtered
off and washed thoroughly, thus removing all of the impurities. The
washed magnesium salt is stirred into 500 cc. boiling water and the mixture
is treated with 15 grams of soda ash, which precipitates the magnesium
as the carbonate and dissolves the dye as the sodium salt. The
hot solution is filtered, washing the magnesite with water, and the Congo
red is salted out by adding 15 per cent (by volume) of salt to the filtrate.
The dye is precipitated as a light red solid which, after drying,
weighs about 70 grams. |
It employs quite a different coupling procedure than the Kline paper, but it uses a
purification which exploits the low solubility of the magnesium salt of congo red in water, which seems like it would be of use here. It appears that
this was the method which was used on a more industrial scale when the book was published, as I believe that was the original target audience of the
book. I suppose I'll let you figure out how to purify the congo red before I need to make benzidine to run the reaction myself Nice job by the way with the procedure. Why was your naphthionate solution so dark,
it appeared in your other pictures that it was similar in color to mine, i.e. light pink. Did that acid to dissolve to give that dark solution? Nice job by the way with the procedure. Why was your naphthionate solution so dark,
it appeared in your other pictures that it was similar in color to mine, i.e. light pink. Did that acid to dissolve to give that dark solution?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by gdflp  | | Why was your naphthionate solution so dark, it appeared in your other pictures that it was similar in color to mine, i.e. light pink. Did that acid
to dissolve to give that dark solution? |
I don't know why it turned dark after the addition of the 5% NaOH. As you say the acid itself was an off-white pinkish color.
Thanks for pointing me to the Fundamental Processes of Dye Chemistry method. I don't know why I didn't check this reference myself. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Wow, the first Congo red thread?
The Manufacture of Dyes gives a couple large-scale processes, representing the large excess and not so much methods. Cain says on page 65: It
has been found that an increased yield of the dye is obtained by using a large excess of sodium naphthionate
A smaller scale preparation is in another good old dye book, which the 1937 Gattermann has translated and halved on p. 302...BTW the 43rd edition
(1982) keeps the synthesis unchanged...except for the reference footnote being replaced by The Obligatory Benzidine Warning.
[Edited on 5-6-2016 by S.C. Wack]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for that information SC.
I don't know that there is any propensity for the diazonium compound to couple with itself. Swamping it with naphthionate would minimize any such
tendency.
0.83g of Congo Red was removed from the filter paper. If pure this would be a yield of 50.3%.
I will attempt to recover more using the MgO method. High temperature is limiting my time in the lab.
[Edited on 5-6-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW some more, though the Organikum dropped this sometime between the 6th and 21st editions, the 5th claims 80% yield from their process that uses
equal amounts.
1 mole of primary aromatic amine is dissolved in 2 1/2 - 3 moles of semi-concentrated hydrochloric acid...in a flask or beaker and, with stirring
and cooling (mixture of ice and salt) at a temperature below 5°C, the amount (In the diazotization of polyamines, the amounts must be calculated
proportionally to the number of amino groups.) equivalent to the amine of a 2.5-molar aqueous solution of sodium nitrite is run in slowly; the
temperature is not allowed to rise above 5°C. Towards the end of the addition of the nitrite, a test is made with starch-iodide paper for the
presence of free nitrous acid (spots, blue coloration). Nitrite is added until the test is still positive 5 min after the addition. Any excess of
nitrous acid, which can interfere with subsequent reactions, is eliminated by the addition of a little urea or sulphamic acid...
A diazonium salt solution prepared from 0.1 mole of amine is added with cooling and stirring to a solution of 0.1 mole of the coupling component in
the equivalent amount of N mineral acid or, in the case of amino-acids, the equivalent amount of N caustic soda solution at 5-10°C. The dyestuff is
separated from the acid dye solution in the form of the dye salt by neutralization with sodium carbonate and salting out with common salt. Depending
on solubility, the dye can be recrystallized from either a little water or a mixture of water and alcohol.
Armarego & Perrin says recrystallize from 1:3 aq. ethanol.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The purification procedure from Fundamentals Processes of Dye Chemistry by Fierz-David & Blangey using MgO is good I think. I used it on
what was leftover from my purification based on Lubs. The picture below is the Mg Congo Red precipitate:

The picture below shows the recovered Congo Red from the MgO process in the flask. As you can see there isn't much Congo Red as the liquid is very
dilute. This is quite contaminated with Na2CO3 and I will discard it. That's the only trouble with the MgO method, ie, it still leaves your product
contaminated with a sodium salt. It does remove organics and other contaminants, however.
The small vial is my ending yield of 0.83g or 50.3% from the Lubs procedure. It is a very dark red, almost black, powder.
The two test tubes show the indicator colors for Congo red: violet for acid, orange for base.

[Edited on 8-6-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unionised
International Hazard
    
Posts: 5134
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | ...This is quite contaminated with Na2CO3 and I will discard it. That's the only trouble with the MgO method, ie, it still leaves your product
contaminated with a sodium salt. It does remove organics and other contaminants, however.
...
|
I'd have a go at extracting the dye from the soda with alcohol, before I discarded it.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Great job Magpie, and excellent illustrated write-ups.
With your first-hand experience of the entire process, what would you describe as the procedure you'd follow on the Next synthesis of Congo Red in
order to avoid the pitfalls you've encountered ?
Still no mothballs here yet, but when they arrive, they will be put through your process.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@aga:
I recommend that you follow the Kline procedure from the Journal of Chemical Education, linked by DJF90.
You can read my posts to see where I had problems.
Making Congo Red is a long journey - taken one precursor at a time. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
It was great, again, Magpie!
Accidentally I found a brief description of Congo Red in one of my org. chem. books a few days ago. It said that the inventor in the 1880-ies (or so)
used very long coupling times between the bis-diazo-benzidine and naphtionic acid. (24 hours!)
It was theoretized that such long times are required because in the case of bis-diazo compounds the coupling between the first diazo group and the
amine is quick, but the second diazo group couples much more sluggishly.
I know it is over, but it might worth considering next time.
So many possibilities, so many variables and so little time.
|
|
|