| Pages:
1
2 |
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
NG/EGDN mixture - freezing curve
Hello there!
Does any of you guys have or knows where I could find a relatively up to date freezing curve/ diagram (temperature versus composition) of a NG/EGDN
mixture?
For clarity sake, it should look something like the one below (probably):
I would be gratefull for any help.
Best regards.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Welcome to scimad!
EGDN addition can lower the FP of NGl to ~-60°.
One might assume the ratio then to be in the range 50/50, but without a graph, it's just conjecture . . .
But EGDN alone should suffice in any cold conditions short of the arctic!
|
|
|
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Hi hissingnoise,
Thank you for your kind welcome 
As far as I know, the 60:40 ratio is the most optimal one, but a graph would definitely help to confirm this.
Also, I'm asking this, because knowing the freezing curve of BG/EGDN one can estimate both the safest and most economical ratio of these two. NG is
much cheaper than EGDN and it has much lower vapor pressure (it is less volatile), therefore it is safer for workers (of course not in terms of
overall toxicity, but at keeping the TLV below limits). Altering the ratio "just a bit" in favour of NG, gives huge savings, but I it cannot be done
without knowing the freezing curve. Safety first.
I was hoping that some of you guys may have seen this curve somewhere or even done such measuring. The only one I saw was over 100 years old  Another one from different source and done more recently, perhaps would not change
anything, but at least I would get some confirmation that the old one is correct. Another one from different source and done more recently, perhaps would not change
anything, but at least I would get some confirmation that the old one is correct.
Cheers.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Bender84,
1°) Glycerol (propantriol) is normally more or equally expensive than glycol.
What are your arguments in favor of a cheaper NG price vs EGDN one?
2°) If you make a mix of the two, you may think that by the colligative laws, you will reduce the volatility of the two compounds...unfortunately it
only works if one of the component is a non volatile compound...so here by opposition you will have a slighly higher vapour pressure of the most
volatile component above it...what changes nothing for security or safety.
3°) Also a more volatile compound in the mix will favor tiny bubbles formation inside it what is a call for disaster in the case of NG and EGDN due
to the increased shock sensitivity.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Glycerol (propantriol) is normally more or equally expensive than glycol. |
And EGDN is attractive to any hobbyist not making industrial quantities or stuck in the tropics ─ its lower viscosity giving higher brisance than
NGl and its zero OB higher strength!
Added to its easy nitration is its lower sensitiveness, which kinda makes EDGN hard to beat!
Pity about the minor downsides of higher volatility and solubility . . .
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
As I recall the vapor pressure of EGDN is only slightly higher (5% or something) than NG, I've seen EGDN residue on glassware that didn't evaporate to
any noticeable degree in over a week. I don't think it's something worth considering other than in determining how a mixture of NG/EGDN would change
over a long period of time due to evaporation.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@All,
Stop recalling and spreading wrong infos!
Check pages 223 to 227 of Rudolph Meyer-Explosives 6th edition. There you find all info about performances, sensitivity and volatility!
RM-6th ed
EGDN is about as sensitive as NG!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Stop recalling and spreading wrong infos![sic] |
Really PHILOU, and what do you see wrong with the info given?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Even with the link provided you don't see?
OK...here it comes...
From you:
" its lower viscosity giving higher brisance than NGl and its zero OB higher strength!
Added to its easy nitration is its lower sensitiveness, which kinda makes EDGN hard to beat!"
1°) It is not the lower viscosity that implies higher brisance but a mix of parameters and most of all the better OB (closer to 0) providing a better
energy output per kg...
Kast introduced the concept of “brisance value”, which is the product of loading density, specific energy and detonation rate.
Brisance EGDN = 1.48 g/cm³ * 7300 m/s * 121 mt/kg = 1307284
Brisance NG = 1.591 g/cm³ * 7600 m/s * 106.6 mt/kg = 1288965
2°)Impact sensitivity NG = 0.2 N m (so falling weight of +/- 20g from 1 m or 200g from 10 cm!)
Impact sensitivity EGDN = 0.2 N m
So what lower sensitiveness?
From OneEyedPyro:
"As I recall the vapor pressure of EGDN is only slightly higher (5% or something) than NG"
Vapor pressure in mbar as function of T(°C)
@20°C vp NG =0.00033
@50°C vp NG = 0.0097
@80°C vp NG = 0.13
@90°C vp NG = 0.31
@0°C vp EGDN =0.006
@20°C vp EGDN =0.05
@40°C vp EGDN =0.35
@60°C vp EGDN =1.7
@80°C vp EGDN =7.8
@100°C vp EGDN =29
--> EGDN is 151 times more volatile than NG (15100%) at 20°C and 60 times (6000%) more volatile than NG at 80°C!
This is far from "slightly higher" and from "5% or something"
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
That is quite a difference  , I'm not sure why I thought they were so similar in
terms of volatility... Still, the evaporation rate of EGDN is fairly insignificant. Many seem to be under the impression that it evaporates quickly
like water when that is just not the case , I'm not sure why I thought they were so similar in
terms of volatility... Still, the evaporation rate of EGDN is fairly insignificant. Many seem to be under the impression that it evaporates quickly
like water when that is just not the case . .
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
PHILOU, it's a bit weird to be arguing with a Brussels resident over the properties of high explosives at this juncture, but bullshit all you want ─
I'm sticking with TL Davis and T Urbanski who, as you should know both contradict Meyer!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Only thing i can add is that 'freezing curve' would be a part of the 'phase diagram' for the substance/mixture.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by hissingnoise  | PHILOU, it's a bit weird to be arguing with a Brussels resident over the properties of high explosives at this juncture, but bullshit all you want ─
I'm sticking with TL Davis and T Urbanski who, as you should know both contradict Meyer!
|
I didn't know but I must say that all those books contains a lot of errors...
Not more than a week ago, I was reading Urbanski Vol 4 and I was horrified by the mistakes made in formulas and equations...I found those by
retro-calculating from tables to come back to the equations but there was always something wrong...then I checked the reference texts and found the
mistakes...
Meyer has also mistakes, I don't understand that they don't ask me  or a real
chemist to check for their 6th edition (I have the 4th in paper book bought with my pocket money at the age of 19 and the same mistakes comes
back)...maybe I should propose to peer review it or a real
chemist to check for their 6th edition (I have the 4th in paper book bought with my pocket money at the age of 19 and the same mistakes comes
back)...maybe I should propose to peer review it 
But Meyer works with numerical data from german or conventional international testings...so I'm more keen to follow their point of view.
Also why would methyl nitrate be very sensitive; then why would ethyleneglycol dinitrate be unsensitive; and then again propantriol trinitrate be
sensitive... while passing from n= 1 to 3 in H-(CHONO2)n-H?
H-(CHONO2)1-H = CH3ONO2
H-(CHONO2)2-H = O2NOCH2-CH2ONO2
H-(CHONO2)3-H = O2NOCH2-CHONO2-CH2ONO2
for Meyer all 3 display IS= 0,2 Nm
[Edited on 28-3-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Hello all,
Thank you very much for the answers 
@PHILOU
| Quote: |
Glycerol (propantriol) is normally more or equally expensive than glycol. What are your arguments in favor of a cheaper NG price vs EGDN one?
|
Glycerol is about 25% cheaper than glycol in EU mainly due to production of biodiesel. It is created during hydrolysis of fats and it is treated as
waste in the process.
| Quote: |
If you make a mix of the two, you may think that by the colligative laws, you will reduce the volatility of the two compounds...unfortunately it only
works if one of the component is a non volatile compound...so here by opposition you will have a slighly higher vapour pressure of the most volatile
component above it...what changes nothing for security or safety.
|
Correct me if I’m wrong, but I think that you can treat NG/EGDN as an ideal mixture, therefore it is subject to Raoult’s Law. So, e.g. for 60:40
mix, the total vapor pressure of the mixture would be 0,0202 mbar (using the vapor pressure values given in Meyer's Explosives 6th ed.), and for the
70:30 mix it will be 0,0152 mbar. You get ~25% decrease in volatility if you increase the NG content by 10%.
| Quote: |
Also a more volatile compound in the mix will favor tiny bubbles formation inside it what is a call for disaster in the case of NG and EGDN due to the
increased shock sensitivity.
|
I have never seen such phenomenon in my career, neither the people who work decades longer than me in the industry. In conditions we are using the
mixture not such thing has ever happened.
And what about the freezing curve? Do you (or anyone who reads this topic) know where I can find such diagram?
Best regards.
[Edited on 29-3-2016 by Bender84]
[Edited on 30-3-2016 by Bender84]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Freezing Points of NG-EGDN Mixtures
I'll take a shot at this.
I have in the past looked for information about the freezing points of different NG-EGDN mixtures and found very little. I spent a bit of time in the
last few days searching the internet and have found a few bits of information to get us started on a phase diagram. Some additional freezing point
values from reliable sources and/or a freezing point constant for EGDN would help if anyone can find them before I do.
From, "Rock Blasting and Explosives Engineering" (pg. 75):
"
Adding EGDN to NG lowers the freezing point from that of NG from 3C to 13C to -25C to -60C dependent on the mixture ratio."
So, -60C is likely the eutectic point (temperature corresponding to the lowest freezing point mixture) or very close to it.
From Meyer:
NG solidification point: 13.2C
EGDN solidification point: -20C
According to "Nitro-Explosives: A Practical Treatise", technical NG has a melting point normally around 10-11C, and sometimes much lower even, because
of the presence of a small amount of di-nitroglycerin. Technical EGDN would of course have a lower freezing point as well because of impurities. We
will use the value of 13.2C for pure NG and the value of -20C for pure EGDN for the phase diagram however.
From, "Nitro-Explosives: A Practical Treatise":
"
According to Raoult's law, the lowering of the freezing point caused by _m_ grms. of a substance with the molecular weight M, when dissolved in 100
grms. of the solvent, is expressed by the formula: [Delta] = E(_m_/M), where E is a constant characteristic for the solvent in question. The value of
E for nitro-glycerine was found to be 70.5 when calculated, according to Van't Hoff's formula, from the melting point and the latent heat of fusion of
the substance. Determinations of the lowering of the freezing point of nitro-glycerine by additions of benzene, nitro-benzene, di-nitro-benzene,
tri-nitro-benzene, p.-nitro-toluene, o.-nitro-toluene, di-nitro-toluene, naphthalene, nitro-naphthalene, di-nitro-naphthalene, ethyl acetate, ethyl
nitrate, and methyl alcohol, gave results agreeing fairly well with Raoult's formula, except in the case of methyl alcohol, for which the calculated
lowering of the freezing point was greater than that observed, probably owing to the formation of complex molecules in the solution. The results show
that, in general, the capacity of a substance to lower the freezing point of nitro-glycerine depends, not upon its freezing point, or its chemical
composition or constitution, but upon its molecular weight. Nauckhoff states that a suitable substance for dissolving in nitro-glycerine, in order to
lower the freezing point of the latter, must have a relatively low molecular weight, must not appreciably diminish the explosive power and stability
of the explosive, and must not be easily volatile at relatively high atmospheric temperatures; it should, if possible, be a solvent of
nitro-cellulose, and in every case must not have a prejudicial influence on the gelatinisation of the nitro-cellulose."
We now have a freezing point constant for NG of 70.5 to use with Raoult's law for freezing point depression.
More to come........
[Edited on 2-4-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by OneEyedPyro  | | As I recall the vapor pressure of EGDN is only slightly higher (5% or something) than NG, I've seen EGDN residue on glassware that didn't evaporate to
any noticeable degree in over a week. I don't think it's something worth considering other than in determining how a mixture of NG/EGDN would change
over a long period of time due to evaporation. |
I guess a possibility is that the stuff you saw not to evaporate was not EGDN but either an impurity or a decomposition product. Stains often look
like they contain a lot of material while they actually don't. Compare it with carbonate stains after tap water evaporates, carbonate is there in ppm
amounts, but gives a clear stain. I can imaging oily residues give the same effect after EGDN evaporates.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
NG-EGDN Freezing Curve
Apparently the equation used is generally most accurate when the solvent (in this case NG) is only slightly diluted, which should be taken into
account when considering the accuracy of the blue curve on the right of the eutectic point. The red curve on the left was not made from values
calculated using Raoult's law, instead it was made in proportion to the blue curve on the right, which should also be taken into account when
considering the accuracy.
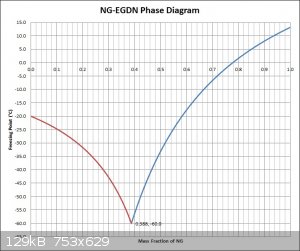
Attachment: NG-EGDN Equilibrium Diagram & Spreadsheet.xlsx (50kB)
This file has been downloaded 582 times
[Edited on 3-4-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Well done Hennig, it certainly looks right and is nearly a mirror-image of Bender's attempt . . .
But one would have to assume that to make any savings in that area, very large quantities would be involved!
Interesting . . . ?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thank you. I wanted this information myself, especially seven or eight years ago or so when I did most of my NG and EGDN experimenting, but never put
much energy and time into tracking it down. Now seemed like a good time.
Edit:
Also, I should have called the graph a freezing curve or equilibrium or phase diagram not freezing points since it is a continuous curve with an
infinite number of points. I just fixed the title of the graph and the last posts title.
[Edited on 3-4-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
NG/EGDN Vapor Pressure Data, etc
The following document contains vapor pressure data, on page four, for NG and EGDN at 30, 40 and 50C as well as calculated vapor pressure values for
10, 20 and 30% EGDN in NG/EGDN mixtures at those temperatures. I didn't bother so far, but it could be useful for someone wanting to make comparisons
and see how similar results from Raoult's law are in this case. Raoult's law may be what was used to obtain the calculated values in the document
anyway. The values could probably be used to obtain a reasonably accurate curve/equation too.
Attachment: Characteristics of Non-Military Explosives.pdf (2.9MB)
This file has been downloaded 869 times
[Edited on 3-4-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Thank you, Henning Brand! 
This helped a lot! Today we have discussed your results. We agreed that your calculations are close enough to the phase diagrams we own  It also corelates well withour experience and knowledge. Unfortunately it also
ultimately dashed our hopes to increase the NG content above 60% It also corelates well withour experience and knowledge. Unfortunately it also
ultimately dashed our hopes to increase the NG content above 60%  Above that
concentration, the mixture has a relative high freezing temperature. Increasing the content of EGDN is not an option due to the high volatility of the
substance (risk of exceeding the TLV). Above that
concentration, the mixture has a relative high freezing temperature. Increasing the content of EGDN is not an option due to the high volatility of the
substance (risk of exceeding the TLV).
Anyways, I would like to share with you one of the two diagrams we own. Perhaps I will be able to post the second diagram later. I had to digitize it
due the bad quality of the scan I received. It is not the one that is 100 years old. This diagram was done more recently by Polish scientist prof.
Syczewski.
Best Regards.
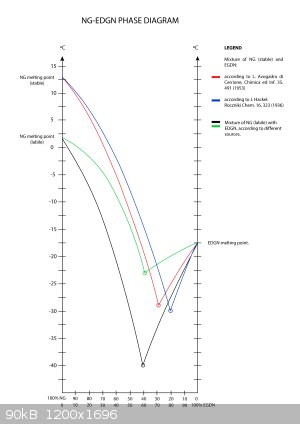
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Bender84  |
@PHILOU
| Quote: |
Glycerol (propantriol) is normally more or equally expensive than glycol. What are your arguments in favor of a cheaper NG price vs EGDN one?
|
Glycerol is about 25% cheaper than glycol in EU mainly due to production of biodiesel. It is created during hydrolysis of fats and it is treated as
waste in the process.
|
True that biodiesel has reduced the price of glycerol down to the price of ethylene glycol between 2003 and 2006.
Maybe that by now the price is lower...I have no datas about it.
But the price will not stay so because vegetable oils (triglycerides) that are used to make biodiesel will be in competition with its use as food...
The EU wishes that biodiesel enters by 10% into the composition of car diesel by 2020 or so...but this is a wild dream because for example for the
tiny country I live in what is Belgium, this would mean to make monoculture of oilish plants on all the Belgium land surface...and this is not
possible, so it has to be done out of the EU in Africa and this would be a disaster for ecology and bio-diversity...but also not possible; so the only
way is to take low price vegetable oils normaly used for the food...thus meaning an increase of both prices diesel and food!
Between low cost food and low cost biodiesel, the people will make a fast choice.
Quote: Originally posted by Bender84  |
| Quote: |
If you make a mix of the two, you may think that by the colligative laws, you will reduce the volatility of the two compounds...unfortunately it only
works if one of the component is a non volatile compound...so here by opposition you will have a slighly higher vapour pressure of the most volatile
component above it...what changes nothing for security or safety.
|
Correct me if I’m wrong, but I think that you can treat NG/EGDN as an ideal mixture, therefore it is subject to Raoult’s Law. So, e.g. for 60:40
mix, the total vapor pressure of the mixture would be 0,0202 mbar (using the vapor pressure values given in Meyer's Explosives 6th ed.), and for the
70:30 mix it will be 0,0152 mbar. You get ~25% decrease in volatility if you increase the NG content by 10%.
|
The mix is not as ideal as you may think it is...because EGDN is a central symetrical molecule with a dipole moment close to 0 Debye while NG which is
a mirror symetrical molecule displays a dipole moment of 3,82 Debye...so you mix something fully apolar with something moderately polar.
Your example is a good one but you only watch at what you want...
If indeed by using 60/40 and 70/30 mixes of NG/EGDN, you (hypothetically by using Raoults law for ideal mixes) reduce the volatility respectively of
59,604% and 69,538% vs EGDN; ab contrario you increase the volatility by 6121% and 4615% vs NG... you noticed it by writing in your last post
"Increasing the content of EGDN is not an option due to the high volatility of the substance (risk of exceeding the TLV)."
Quote: Originally posted by Bender84  |
| Quote: |
Also a more volatile compound in the mix will favor tiny bubbles formation inside it what is a call for disaster in the case of NG and EGDN due to the
increased shock sensitivity.
|
I have never seen such phenomenon in my career, neither the people who work decades longer than me in the industry. In conditions we are using the
mixture not such thing has ever happened.
|
It must be linked to the solubility of gases into NG and EGDN that must be different so saturated solution at STP when mixed will degas from the
solvent that dissolves the more due to the solvent that dissolves less.
Owing to the very different dipole moment, one may expect very different solubilities.
The tiny bubbles will be enriched by the more volatile solvent --> EGDN.
Quote: Originally posted by Bender84  |
And what about the freezing curve? Do you (or anyone who reads this topic) know where I can find such diagram?
|
About the freezing and vaporisation curve I'm working on it but I must admit that the fact NG displays two solid forms with two different MPs (labile
NG=3°C and stable NG 13°C) is not easy.
Also I like the effort of Hennig Brand, but the Raoult's law for lowering of the MP is only valid if dilluted so close to the edges.
I'm checking the calcul spreadsheet of Hennig to understand it and see where problems my be...
dT = Kc * m
Where:
1°)Kc is the cryoscopic constant (lowering of mp in°C for 1 mole of undissociated solutant in 1 kg of solvant)
2°)m is the molality thus the number of moles (n) of undissociated solutant in 1 kg of solvant = n/kg
By definition n = mass of solutant/ MW solutant = m/M
dT = Kc * m = Kc * n/kg = Kc * (m/M)/kg
If n=1 --> m=M and dT = Kc
So the dependance between variation of T° and m (or n of solutant or m of solutant) should be linear with a slope of Kc but the graph of
Hennig is not linear...
[Edited on 8-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Bender84, thank you for the kind words and thanks for providing the graph as well. Very interesting!
Yes, it looks as though the intermolecular forces between NG and EGDN are very different than between molecules of NG.
The amount of solvent is fixed, so the temperature drop does not change linearly with mass fraction / percentage NG or EGDN.
I will have another look at this in the next few days if I get a bit more time.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes Hennig, you have used a mass fraction...
Usually Raoult's law for vapour pressure plays with X = the mole fraction.
For a binary system A and B...
Xa = na/ntotal = na/(na+nb)
By definition Xa + Xb = 1
Indeed when the solvent is in large excess vs solutant, then mass fraction, molar fraction, molality do display similar...
The main problem is that the effect of lowering of the melting point applies to both compounds here since they interact on each other...and this
especially in the middle regio where the two compounds are 50/50.
This effect is used for a fast test in organic lab to be sure of the identification of a compound of known melting point.
If you have two solid compounds A and B with similar melting points, A is your reference compound and B is the one you want a verification of.
Then you put A, B and M (a A/B mix (50/50)) in 3 separate capillarity tubes and you put those in a metal heating block to check the simultaneous
melting point/temperature of A, B and M.
If M melts at the same time as A and B; then A and B are identical;
but if M melts prior to A and B; then A is a different molecule than B.
[Edited on 9-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
[Delta] = E(_m_/M)
where,
[Delta] = temperature change for 100g of solvent
m = mass of solute
M = molecular weight of solute
E = Constant (70.5 for NG)
or
dT = Kc * (m/M)/kg
Divide E = 70.5 by 10 to give Kc = 7.05 and we have the same equation, except now it measures temperature change for 1kg (1000g) of solvent not 100g
anymore.
The first way above, from Nitro Explosives, is likely not conventional anymore, but I think it gives the same results.
I didn't intend for what I did to be the final word on NG/EGDN phase diagrams. I found a few bits of information on the internet, and got a start on
it. Though probably very inaccurate, it was still a very worthwhile exercise because of what was learned/is being learned.
[Edited on 10-4-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
2 |