| Pages:
1
2 |
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Glycine diazotisation in HCl - blue nitroso product?
At the recommendation of a very helpful ScienceMadness member we tried out the process outlined in Weygand and Hilgetag on page 256 entitled
"Replacement of NH2 in aliphatic compounds by halogen by means of a nitrosyl halide."
Instead of alanine we used glycine (5g) as the alpha-amino acid dissolved in an excess (60ml) of 6M HCl. This was chilled to 0C and stirred
vigorously, and finely crushed sodium nitrite (1.4 molar ratio to the glycine) was adding to the mixture very slowly keeping the temp below 5C using a
salt-ice bath.
On addition there's a fizzing, an immediate yellow colour, and yellow gas (also some brown NOx but definitely yellow nitrosyl chloride) being
generated. After a few minutes there was also a characteristic green/azure blue colour of nitrous acid being formed in the mixture. At the end of 1
hour addition time, the mixture was stirred at 0C for another 2 hours then slowly allowed to warm to room temp (about 15C) over another hour. The
reaction mixture at the end of this time was bright clear blue - just like a strong copper (II) sulfate solution!
This was extracted with 25ml of ether and the blue colour passed into the ether layer. On separating, drying with MgSO4, and evaporation this left
about 3ml of a bright blue clear liquid; not viscous at all - like water. Interestingly whilst evaporating off the ether, the blue colour had the
weird property of 'climbing out' of the petri dish and soaking the area around it! It's not that volatile; we left in the breeze outside for 2 hours
and a bit has gone but it's still mostly there.
It's got a slight 'NOx aroma' but we didn't sniff it too hard because it seems to be quite nasty. On cleaning up the area around the evaporating dish
I got a small smear of it on a finger (the liquid immediately went though a latex glove!). Despite immediately washing off and scrubbing with soap,
the skin was dyed an intense blue colour (looks like a blue pen leaked). 3 minutes later there was a twinge of irritation , the skin seemed dry and
and then you could actually watch and see in real-time the slow spread of the skin turning white and flaky and dying... So it's real nice and friendly
y'all.
So we definitely haven't got chloroacetic acid. But does anyone know what this could be? Could this be an alkyl nitroso compound? (We read that these
can be bright blue in colour.)
Any thoughts appreciated.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
When an amine is vicinal to an EWG the reaction with nitrous acid produces a diazo compund:
https://en.wikipedia.org/wiki/Diazo#From_amines
This is because the diazonium salt is acidic enough to protonate hydroxide rather than undergo Sn2. In the case of glycine complex mixtures are
obtained:
http://www.jbc.org/content/82/3/587.full.pdf
I hypothesize that glycine + HNO2 first forms diazomethane, which undergoes further reaction with nitrous acid and water to form formaldehyde and
methyl nitrite, which can react with additional diazomethane in multiple ways to give a mixture of products. It's hard to imagine what's preferred.
[Edited on 10-3-2016 by clearly_not_atara]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
DOI 10.1039/CT8956700489: Nitrosyl chloride (anhydrous) formed chloroacetic acid with glycine ("glycocine").
The Weygand and Hilgetag analogous preparation using alanine stirred longer and did not mention warming to room temperature before extraction; I don't
know if that influenced your result. I apologize if I've sent you on a wild goose chase.
In aqueous solution the glycine reaction in particular has been studied and found complicated; see DOI 10.1039/JR9500000149.
Quoting:
The reaction solutions always developed a green colour, which was more intense the lower the temperature and the higher the nitrite-ion
concentration. It was at first thought that methylnitrosolic acid (ON*CH:NOH, from HONC and HNO2) was the cause of the colour. This acid is
known to be green and to decompose to formohydroxamic acid (Wieland and Hess, Ber., 1909, 42, 4175). However, methylnitrosolic acid is easily
extracted by ether (to give a green extract), whereas the green colour in the glycine, nitroacetic acid, and methylnitrolic acid reaction solutions
could not be removed in this way. Therefore, it appeared that the colour might be that of the anion of a more acidic nitroso-compound. The moderately
stable green colour was obtained irrespective of the acid used (hydrochloric, sulphuric, acetic). When, however, hydrochloric acid was added in excess
of that required for reaction the green colour became deep blue. This colour could easily be extracted by ether. On drying of the ethereal extract the
colour changed to emerald-green. Evaporation of the ether under reduced pressure yielded a green oil, which was explosive at room temperature, and
from its reactions was considered to be a chloronitroso-compound. The green oil becomes blue when added to water, and, on addition of alkali,
olive-green. These colour changes are reversible. This suggested that the green colour of the reaction solution might be caused by the ion of the
chloronitroso-compound, ON*CCl:NO- (which would be stabilised by resonance). With sulphuric and with acetic acid the anions formed would be
ON*C(HSO4):NO- and ON*C(OAc):NO-, respectively. It is assumed that in these cases the free (un-ionised) acids are too
unstable to exist in visible concentration. [Nef found that CHCl:N*OH is much more stable than CH(HSO4):N*OH ; Annalen, 1894,280, 316.] The
blue colour of the aqueous reaction solutions and the behaviour of the green oil were observed whenever hydrochloric acid was used in the treatment of
glycine, nitroacetic acid, or methylnitrolic acid with nitrous acid. The gas evolutions were, however, independent of the acid employed.
PGP Key and corresponding e-mail address
|
|
|
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Thank you for that, and you're right, looking back we didn't follow the original procedure right in allowing it to warm up. We got that wrong.
But it looks from your link as though this isn't the problem; as you say, glycine reacts in a different way. But it's actually very fascinating
nevertheless - the suspected deep blue chloronitroso compound is easily isolated using ether as the reference describes. Obviously our product was
still wet (deep blue rather than emerald green).
Unfortunately the 'file attachment' function on the reply system doesn't work (if I select an image it then hangs if I try to post the response),
otherwise I have a nice photo of the product demonstrating the blue colour.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Is the file perhaps very large? Try shrinking it down to e.g. an image no more than 800 pixels at the largest dimension. The file attachment function
gets used all the time and as far as I can tell it's still working.
PGP Key and corresponding e-mail address
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
No need for a picture. We did a video on the reaction. Very pretty but particularly nasty...
https://www.youtube.com/watch?v=H8lon0TyPl0
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
That is the colour of diazomethane, I believe. How has your health been, chemplayer, and the chemical burn?
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
The 'burn' was a few months ago when we did this originally (see thread above) and it was fine after a few days - but scary to begin with and alarming
that it can go through gloves in the blink of an eye. There was plenty of ventilation in case of NO2 production, so we didn't even get to smell the
reaction mixture.
Not convinced by the diazomethane argument though. The reaction looks identical to the yellow vapour generated when strong hydrochloric acid meets
sodium nitrite, which is nitrosyl chloride. Apart from chucking in a lit match from a few hundred yards away, how would one go about testing to see if
there was any diazomethane present?
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Chemplayer, not diazomethane itself. It is too reactive to be your compound, but a derrivative. Maybe diazoaceticacid.
Anyway, good to hear that you haven't prematurely ended your career.
I wonder if your blue liquid would participate in a Sandmyer type reaction, with, say, Cu2I2? It should be obvious by the disappearance of the blue
and by nitrogen fizzing out.
|
|
|
smerg
Harmless

Posts: 5
Registered: 3-6-2016
Member Is Offline
Mood: No Mood
|
|
chemplayer you rock i love your channel!
One comment:
I do not believe you have any diazomethane in your product. it is a gas at room temperature. it is highly toxic and exposure leads to death. you
cannot generate it unless you have a n-methylamino compound in there which is subsequently n-nitrosated and exposed to base.
a few questions:
1. Is the color change after adding the product to base reversible if you added acid back? does it indicate pH or react?
2. does the product escape from or display noticable capillary action in a plastic or teflon container? This stuff reminded me of the Helium
superfluid video!
3. what if you tried generating nitrosyl chloride as a gas and bubbling it into a glycine solution? would it yield the same product? i suspect that
nitrosyl chloride does not react with glycine...
4. if you used sulfuric acid is the product the same?
just stuff to think about. your the man keep up the good work!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Could it be that diazoacetic acid or a derivative is inthere?
I know that N2CH-CO2H exists as an ester or salts with very interesting chemistry.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This reaction indeed is quite remarkable. I tried the following:
Dissolve some glycine in water.
In the same water, also dissolve some NaNO2.
When this is done, no visible reaction occurs, a colorless solution is obtained.
Cool down the liquid and slowly add cold HCl (36%) while stirring. When this is done, then the liquid heats up considerably. The reaction is quite
exothermic. A fairly large amount of a brown gas mix is produced, but not overwhelmingly so. On addition of the acid, I observe a transient
olive-green color, which quickly fades and is replaced by a sky-blue color, much like a solution of CuSO4 in water.
I now have the liquid standing for one day, to see how stable the blue material is. If it still exists after a day I'll try to extract some of it into
ether.
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Woelen, glad you were able to replicate the experiment. At 5:48 in our video you can see that olive green colour forming initially before the 'copper
sulfate'-like blue appears later.
We found that the amount of NO2 produced during the reaction depends on the temperature. If everything is done slowly and kept around zero degrees
then there is a small amount produced, but not a lot.
Be interested to hear if you managed to extract the blue substance using ether. Please be careful as the ether evaporates though as you'll get that
'creeping' effect as the very corrosive liquid starts to seek out world-domination and explores the surrounding area!
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
After 24 hours, the liquid still was bright blue, just as it was when I made it.
I also could extract the blue compound in ether. I had a more dilute solution than you had, my extract in ether was like a moderately concentrated
solution of CuSO4 in water. The aqueous layer became nearly colorless.
I allowed some of the ether to evaporate so that appr. 1/4 part of the liquid remains. I also observed the upwards "creeping" of pale blue liquid.
The liquid is a somewhat deeper blue, like a highly concentrated solution of CuSO4 in water. On standing, the extracted liquid slowly turns green.
After one hour, it has a beautiful green color, much like a concentrated solution of NiSO4 in water. I will allow the green liquid with ether to stand
for a longer time to see how the color develops.
I also added a small quantity of the blue ether solution to a solution of NaOH. This results in formation of a green/yellow solution. When this
green/yellow solution is acidified again, then it becomes colorless (or maybe very pale blue).
I now only made a very small quantity of the blue compound, next weekend I'll try to make more, maybe 5 ml or so. Ampouling this liquid and keeping it
around for a longer time may be interesting, but I am somewhat reluctant to doing this, maybe it cracks/explodes due to pressure buildup in the
ampoule?
[Edited on 27-6-16 by woelen]
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
I would be cautious about ampouling it. We left a small amount of the concentrated blue liquid in a small beaker for a while, and could definitely
detect some nitrogen oxides very slowly coming off. Possibly a reaction with air, but possibly decomposition (a bit like an organic nitrite ester
perhaps).
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It is interesting to see that the green liquid turns paler on standing. The ether evaporated now and what remains is an odorless, nearly colorless
oily liquid. Apparently the green liquid decomposes, leaving a colorless liquid behind.
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
I honestly can't imagine the horrors if a full ampoule exploded while in hand. Also, I saw a Wikipedia page on 2-Methyl 2-nitrosopropane, wherein it
described it being in an equilibrium, a blue liquid for the monomer, and a white/colorless solid for the Dimer. Could this be at all possible? I
highly doubt it, it doesn't make too much sense, but it did catch my attention.
-Azide N3
Link to 2M2NP: https://en.m.wikipedia.org/wiki/2-Methyl-2-nitrosopropane
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did not ampoule any of the blue liquid. It is unstable. I tried two times keeping it around. One time as a (relatively) pure liquid, another time as
a solution in ether. In both cases, after a few days, the blue color fades away, leaving a very pale yellow compound.
The blue compound and the colorless dimer are not that special. Many nitroso compounds are blue. The simplest one is O2N-NO, which also is a deep blue
liquid (you can see this in solution if you add a small quantity of strong acid to an ionic nitrite, dissolved in ice-cold water).
Organic nitrosocompounds can dimerize on the nitrogen atom, or on both the nitrogen-atom and its connected oxygen atom. The dimer of
2-methyl 2-nitrosopropane is even available commercially:
http://www.sigmaaldrich.com/catalog/product/aldrich/180262?l...
[Edited on 14-9-16 by woelen]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nitrosocompounds may dimerize.
It depends onto the rest of the molecule.
2 R-N=O --> R=N-O-O-N=R (peroxyde structure)
2 R-N=O --> R=N-O-N(O)=R (for example furoxane structure)
2 R-N=O --> R-N(O)=N(O)-R (diazo di-N-oxyde structure)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Good that you noticed the error, in my post I indeed meant nitrosocompounds and not nitrocompounds. I'll change the original post.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I bet the mysterious blue compound is the nitrous ester of glycolic acid.
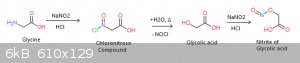
Non-aryl diazonium compounds are very unstable. Theoretically nitrogen is eliminated and leaves a carbocation. This is added to by the HCl to form a
very unstable chloro- intrmediate. However, the nitrite competes in the reaction for the chlorine's electrons, which are relatively free considering
the close proximity to the carboxyl resonance. The length of reaction time is explained by the HCl addition to a 1° center next to a bunch of
electron withdrawing groups, and is probably the slow step. The chlorine is then lost from the compound spontaneously and is picked up by nitrous acid
which has been protonated by the stronger HCl, forming nitrosyl chloride and water. The nitrosyl chloride is nucleophillic toward the vulnerable
center on what's left of the glycine molecule, and attaches to form the chloronitroso compound in step 2.
Chloronitroso compunds are probably yellow in color and also quite unstable, and on warming undergo hydrolysis, eliminating NOCl (which is observed)
and forming free glyoxylic acid. The hydroxyl on the acid is now free to form an ester by deprotonation by excess HCl followed by attack from nitrous
acid, the standard way, leaving the nitrous ester. These esters are typically deep blue/green and unstable. The color you describe is close to nitrite
esters I once carefully prepared from ethylene glycol and glycerol. This new ester is probably stabilized by withdrawn electrons considering the high
degree of resonance on both ends of the molecule.
The description of your injuries sounds like capillary emboli a la hydrogen peroxide, however in this case small bubbles of NOx blocking the
capillaries just under the skin as various enzymes catalyze the destruction of the compound, giving a visible whitening/graying effect that does not
last. This would leave behind glycolic and nitric acids, which would be unpleasant considering glycolic acid is stronger than acetic. The nitric acid
probably contributed to a yellow stain. How long did the blue last? Was it replaced by yellow/orange? Did the whitening/graying disappear in a few
hours?
Of course, I could be completely wrong, but that's my best attempt to describe what happened. You should try the action of nitrous acid on glycolic
acid and see if you get the same blue product.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I believe the nature of the carbonyl group plays a role in how this reaction proceeds. While sodium nitrite and hydrochloride acid react with glycine
to form an unknown blue compound, sodium nitrite and catalytic sulfuric acid in a cold DCM/water biphasic mixture reacts with ethyl glycinate
hydrochloride to form ethyl diazoacetate (a useful stabilized diazomethane equivalent). Ethyl diazoacetate is a yellow liquid at room temperature. See
http://www.orgsyn.org/demo.aspx?prep=cv4p0424 for a reference. What I believe is happening in the ester case is that the electron-withdrawing
carboxylic ester group is making the alpha protons more acidic, such that when the diazonium intermediate is formed, instead of eliminating nitrogen
one of the hydrogens is lost instead to form a diazo compound.
On the other hand, if the reaction pH is not low enough, the carboxylic acid in free glycine may very well deprotonate. The resulting deprotonated
carbonyl is now somewhat electron donating, and therefore discourages the formation of a diazo compound. So, an intermediate diazonium carboxylate
zwitterionic salt is formed, which is open for nucleophillic attack at the alpha carbon. Nitrite is more nucleophillic than chloride, and so it will
add and kick off a nitrogen, forming the nitrite ester of glycolic acid, as well as some potential nitroacetic acid.
On the other hand, if the acid does not deprotonate, the diazo compound should form, and very quickly decompose to a complex mixture (diazoacetates
are unstable to water, but their esters partition away into organic phases). I doubt that compounds of this type are responsible for the color due to
this instability to hydrolysis, but I may be wrong.
In so many words, this is another possible explanation for Praxichys's glycolic acid theory, but I am not convinced that his mechanism is correct. I
do believe there is a test of my mechanism, however. If sulfuric acid is used as acid instead of hydrochloric acid, and if acid is added to the
nitrite mixture, the mechanism I proposed will still be possible (in fact, even more possible, due to the sulfate being even less electrophillic than
chloride, and due to the pH only dropping low enough for the acid to stay unprotonated towards the end), and if the same blue colored material forms
with this reaction, that is some evidence that the halogen's role is at least somewhat unnecessary. I might give this a try in a couple weeks if no
one picks it up before I do.
On the other hand, let me provide some evidence against my theory in the interest of stirring up some more discussion. With amino acids larger than
glycine, this hydrochloric acid-sodium nitrite reaction forms the chloroacid in reasonable yield. However, the stereochemistry is preserved! http://orgsyn.org/demo.aspx?prep=cv8p0119 This kind of indicates to me that something strange is going on, and someone smarter than me needs to
try and figure it out. 
I am on mobile right now, but if anyone is interested this paper has some discussion on the reaction of diazoacetates in aqueous mixtures. http://pubs.rsc.org/en/content/articlepdf/1961/tf/tf96157019...
[Edited on 14-9-2016 by Cryolite.]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If this were the case, then
would you expect formation of a blue compound on addition of nitrite to glycolic acid (either alone, or mixed with HCl)?
I did the experiments. In one experiment I added NaNO2 to 30% or so glycolic acid in water. This leads to slow formation of colorless gas, turning
brown on contact with air. The liquid remains colorless, also after some time.
In another experiment, I mixed some 70% glycolic acid with 36% hydrochloric acid and added a little water as well. When NaNO2 is added to this liquid,
then there is vigorous formation of a brown/yellow gas mix and the liquid turns golden yellow. On dilution with its same volume of water, the golden
yellow color disappears and the liquid becomes colorless.
Finally, I added conc. HCl to the mix of NaNO2 and glycolic acid. When this is done, then the liquid also turns golden yellow, but less intense. On
addition of more solid NaNO2 again, the deep golden color is obtained.
No traces of blue compounds, nor anything green. Also on standing there is no formation of anything blue or green. With the same conditions (same
temperature and similar concentrations of HCl and NaNO2) with glycine I get a blue compound, which turns green on standing.
Based on this experiment, I get the impression that the blue compound is not a glycolic acid ester of nitrite. I even think that the golden yellow
color is not any organic ester at all. On dilution the yellow color disappears. I think that it simply is ONCl, dissolved in the strong acid mix,
which hydrolyses to HNO2 and HCl on dilution with water.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Cryolite.  | On the other hand, let me provide some evidence against my theory in the interest of stirring up some more discussion. With amino acids larger than
glycine, this hydrochloric acid-sodium nitrite reaction forms the chloroacid in reasonable yield. However, the stereochemistry is preserved! http://orgsyn.org/demo.aspx?prep=cv8p0119 This kind of indicates to me that something strange is going on, and someone smarter than me needs to
try and figure it out.  |
I haven't really been following the discussion in this thread, but I can tell you why the stereochemistry is retained in that orgsyn procedure. What
happens is that the electron-deficient alpha-carbon of the diazonium salt undergoes an intramolecular nucleophilic attack from one of the oxygens of
the deprotonated carboxyl group. Keep in mind that an intramolecular attack will just about always occur more rapidly than an intermolecular attack.
This attack cleaves the C-N2+ bond and inverts the stereochemistry of the alpha-carbon, giving a substituted chiral acetolactone
ring.
The unstable lactone is then quickly attacked by a chloride ion, opening the ring back up and inverting the stereochemistry once again. Thus you end
up with retention of configuration and the alpha-carbon in the (S)-configuration.
Anyway, if you're interested, I also drew the mechanism for you:
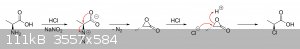
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | Quote: Originally posted by Cryolite.  | On the other hand, let me provide some evidence against my theory in the interest of stirring up some more discussion. With amino acids larger than
glycine, this hydrochloric acid-sodium nitrite reaction forms the chloroacid in reasonable yield. However, the stereochemistry is preserved! http://orgsyn.org/demo.aspx?prep=cv8p0119 This kind of indicates to me that something strange is going on, and someone smarter than me needs to
try and figure it out.  |
I haven't really been following the discussion in this thread, but I can tell you why the stereochemistry is retained in that orgsyn procedure. What
happens is that the electron-deficient alpha-carbon of the diazonium salt undergoes an intramolecular nucleophilic attack from one of the oxygens of
the deprotonated carboxyl group. Keep in mind that an intramolecular attack will just about always occur more rapidly than an intermolecular attack.
This attack cleaves the C-N2+ bond and inverts the stereochemistry of the alpha-carbon, giving a substituted chiral acetolactone
ring.
The unstable lactone is then quickly attacked by a chloride ion, opening the ring back up and inverting the stereochemistry once again. Thus you end
up with retention of configuration and the alpha-carbon in the (S)-configuration.
Anyway, if you're interested, I also drew the mechanism for you:
|
Thanks for that! That double Sn2 mechanism is really pretty to look at. I wonder why it breaks down for glycine though. Maybe the unsubstituted
acetolactone is just too unstable to form even transitionally? Wikipedia says alpha propiolactone is stable, so maybe...
|
|
|
| Pages:
1
2 |