| Pages:
1
2
3 |
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
Willgerodt rxn: Styrene monomer -> phenylacetamide
Information on this reaction is quite difficult to track outside the Hive, did anyone keep notes on it?
It involved styrene monomer, NH3 solution, ethanol, and H2O from memory.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Go here and get organic reactions vol III check out page 97............solo
http://www.sciencemadness.org/library/index.html
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
Thanks a lot solo, it looks like they just used pyridine rather than ethanol...
with ethanol the yield is much higher, in to the excellent range. And from memory the temperature was more like 100C.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
That's the classic reference solo gave,
With aqueuos NH3, and sulfur or sulfer + ammonium sulfide, it must be run under pressure, usually in a sealed tube. It can be run with a high boiling
amine and dry sulfur at atmospheric pressure; under these conditions it forms a thioamide which is hydrolised during workup to the free acid.
No ethanol, except as a solvent for workup; the reaction conditions would convert it to acetic acid and/or boil it off.
http://www.organic-chemistry.org/namedreactions/willgerodt-k...
http://www.chempensoftware.com/reactions/RXN407.htm
http://www.thieme-connect.com/ejournals/abstract/synthesis/d...
improved yields - you'll want the full article, this is just 1st page
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1946/68/i10/...
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1953/75/i01/...
Works OK on simple alphatic ketones too
http://www.znaturforsch.com/ab/v59b/59b0601.pdf#search=%22Wi...
Why don't we do it in the microwave?
http://www.ingentaconnect.com/content/stl/jcr/2000/00002000/...
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
The Willgerodt Reaction
Ellis V. BROWN*
Synthesis pg.358, 1975
Abstract
The Willgerodt reaction is discussed from a synthetic standpoint including types of compounds for starting material and conditions of operation. The
Kindler modification and its usefulness is also indicated. The behavior of heterocyclic compounds in the reaction is mentioned followed by a number of
interesting special compounds prepared. The results obtained by applying the reaction conditions to cyclic ketones are covered and, finally, work on
the reaction mechanism to date is discussed.
1. Introduction
2. Kindler Modification
3. Reaction of Heterocyclic Compounds
4. Special Syntheses
5. Results with Cyclic Ketones
6. The Overall Mechanism
7. The Detailed Mechanism
[Edited on 25-8-2006 by solo]
Attachment: The Willgerodt Reaction.pdf (803kB)
This file has been downloaded 5908 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
The Willgerodt Reaction. 11. A Study of Reaction Conditions with Acetophenone
and Other Ketones
EDWARCDE RWONKA,' K. CHRISTIAN rlNDERSON3 .1ND ELLISv . BROWN
Journal of the American Chemical Society pg 2025, 1946
Attachment: The Willgerodt Reaction.11. A Study of Reaction Conditions with Acetophenone and Other Ketones.pdf (635kB)
This file has been downloaded 2245 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Studies in the Mechanism of the Willgerodt Reaction. 111. Nature of the Labile
Intermediate
Y EDWARCDE RWONKA,' K. CHRISTIAN rlNDERSON3 .1ND ELLISv . BROWN
JACS vol. 53, pg. 30, 1952
Abstract
The nature of the labile intermediate participating in the Willgerodt reaction with phenyl n-butyl ketone has been investigated using deuterium as a
tracer. 6-Phenylvaleramide, the reaction product from this ketone, was found to have retained only 5% of the deuterium which had originally
replaced hydrogen on the beta carbon of the alkyl side chain. This evidence is interpreted as indicating that an unsaturated intermediate has been
formed during the course of the Willgerodt reaction.
Attachment: Studies in the Mechanism of the Willgerodt Reaction.pdf (579kB)
This file has been downloaded 1474 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | No ethanol, except as a solvent for workup; the reaction conditions would convert it to acetic acid and/or boil it off.
|
There is a method using ethanol during the reaction. It was quoted in a Chinese article if my memory serves me correctly and was tested by a member at
the Hive.
The efficacy of it might have been specific to the styrene -> phenylacetamide reaction.
Thanks for your digging and searching you guys!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It's mentioned here http://www.sciencemadness.org/talk/viewthread.php?tid=1745
and is being used as a solvent. Being a patent, it could be someone attempting to get around another patent. Many solvents have been tried in the
past, we tried ethylene glycol but it was oxidised by the reaction conditions. You really need to seal it, to keep the NH3 from escaping, or use a
higher boiling amine.
This might prove interesting
http://taylorandfrancis.metapress.com/(hx1kfyynnvghe545ntomb5e2)/app/home/contribution.asp?referrer=parent&backto=issue,21,21;journal,135,136;link
ingpublicationresults,1:107868,1#search=%22Willgerodt%20%20reaction%20ethanol%22
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
My understanding was that ethanol replaced pyridine in the reaction, and the temperature was approximately 94 or 96 degrees centigrade.
Having said that, there's much more info outside the Hive suggesting that your right.
ORganikum would remember the two discussions im refering to at the Hive.
Anyhow, about the reaction, does anybody know how smelly it is, whether the gases are toxic, and what would be the best method of measuring
temperature of the sealed vessel?
I was thinking an infra-red pyrometer.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Just use a heating bath, the tube won't get hotter than the open bath which is easy to monitor the temperature of.
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
not_important do you have the full text for the microwave-assisted variation?
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | not_important do you have the full text for the microwave-assisted variation? |
The article:
Microwave assisted Willgerodt-Kindler reaction of styrenes
Moghaddam F.M.; Ghaffarzadeh M.; Dakamin M. G.
Journal of Chemical Research, 1 May (2000), pp. 228-229(2)
Styrenes are efficiently transformed to thioamides via the Willgerodt-Kindler reaction under microwave irradiation.
[Edited on 28-8-2006 by leu]
Attachment: moghaddam.zip (32kB)
This file has been downloaded 1651 times
Chemistry is our Covalent Bond
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, now I have the full text. Thank you, leu!
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
https://sciencemadness.org/talk/viewthread.php?tid=1745
See, this guy is working off the same information, Ill try get the patent he mentions translated.
But the numbers he produces aren't what I remember at all, they were more like:
Sulfur 12.5g
ethanol 10ml
styrene 7.4g
NH4OH 10ml
The guy who translated them at the Hive was Chinese I believe, so...
EDIT: actually from looking at the patent I think roger2003 has it right, and 21.7g does ring a bell.
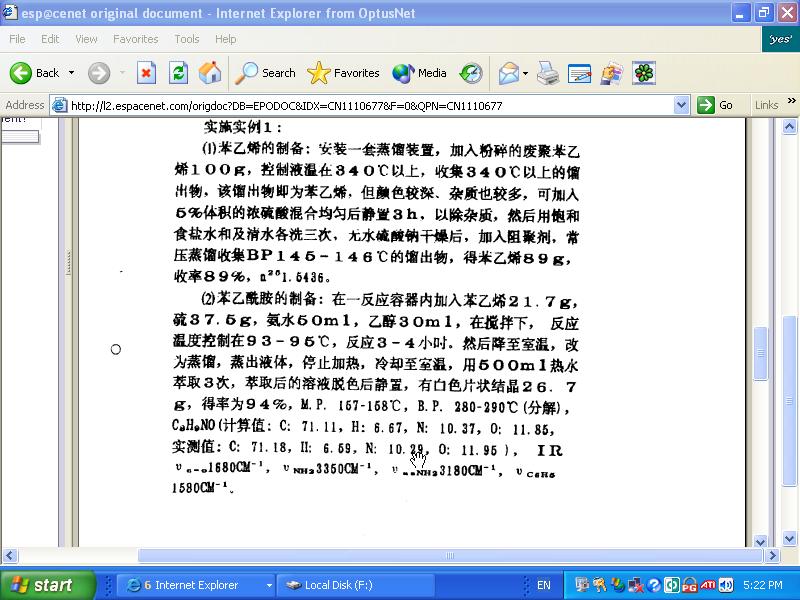
Ill post an image of the description section and hopefully someone who knows Chinese can match the amounts with the compounds.
[Edited on 30-8-2006 by visitation123]
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
Screen shot of majik paragraph
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
OK
I think I've worked it out by translating the english words for the compounds to simplified chinese then matching the characters with what appears on
the patent...
21.7g styrene monomer
37.5g sulfur
50ml NH4OH
30ml ethanol
I think the reaction calls for a reflux but it can be done in a sealed vessel submerged in boiling water for four hours...I know (believe) this
because someone at the Hive claimed to have done so and yielded a red product (phenylacetamide).
The yields are said to be in the 80+% range.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I think this is supposed to happen in a sealed vessel, not at reflux. I recall someone else's translation revealing that the reaction is supposed to
be conducted at 100 C, but that mixture will certainly start boiling at lower temperatures. I've tried it with improvised reflux. I don't think I got
much, but I will say that a uniquely clinging, unpleasant stench is one of the products. I don't know if that is a byproduct or the main show. Who can
describe the odor of phenylacetamide?
[Edited on 8-30-2006 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
As far as the smell Polverone I'm uncertain, but I think that phenylacetic acid can be a by-product of the reaction so it might have been that?
Phenylacetic is said to be a very strong sweet smell AFAIK.
About whether it's reflux or sealed, well sealed would be consistent with most literature on the reaction.
A pressure vessel could be constructed with a gas tank, a short length of copper tubing leading to a ball valve and so that the fluid contents can be
distilled away when the ball valve is in the open position. Hopefully that'd run smoothly. Only one way to find out!
For the work-up, I recall that the reaction fluids are distilled away and that the phenylacetamide is extracted with H2O. But I can't remember if the
H2O was supposed to be heated or rt.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The free acid is a byproduct, from hydrolysis of the amide. Phenylacetic acid has a strong smell, some consider it unpleasant. Its esters are more
enjoyable, many are used in perfumery. (phenethyl alcohol is nice, flowery) The thioamide and/or similar compounds stink, even traces are noticable.
The amide is not very soluble in cool water, even hot water isn't a great solvent; the free acid dissolves well enough in hot water that
recrystallisation from water has been used for purification.
I wouldn't use copper around reactions that use ammonia or the lighter amines, they can greatly speed up corrosion of copper. Even worse when there is
going to be free sulfur and H2S around.
Worse that the solvent boiling is the loss of NH3 if the reaction isn't sealed away. That's why the Kindler modification switched from ammonia to
morpholine; other high boiling amines will work.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by visitation123
A pressure vessel could be constructed with a gas tank, a short length of copper tubing leading to a ball valve and so that the fluid contents can be
distilled away when the ball valve is in the open position. Hopefully that'd run smoothly. Only one way to find out! |
Don't you think you are exaggerating big time. You don't need a hardcore metal autoclave. The pressure stays below 5 bar so you can use simple and
ready made solutions. Search the forum for "pressure vessel"! (Or should I say UTFSE?)
| Quote: | | For the work-up, I recall that the reaction fluids are distilled away and that the phenylacetamide is extracted with H2O. But I can't remember if the
H2O was supposed to be heated or rt. |
Nonsense. As you were already told, phenylacetamide is not water soluble and I'm pretty sure the other main product, phenylthioacetamide, is even less
watter soluble. Most of the sulfur is going to be dissolved as the polysulfide and most styrene either reacted or polymerized. So there should mostly
be organic solids left which should be ready for hydrolysis by a simple filtration and washing. Sulfur and other contamination will not affect the
hydrolysis. If instead a paste is obtained (quite likely), then you would need to extract and purify by recrystallization.
In my experience phenylacetamide barely has any smell which is just a subtle indefinable one. It is a white crystalline powder. I don’t know about
the thioamide. I never had the “pleasure” to work with it. Phenylacetic acid smells bad and the description of “mouse shit smell” that I read
somewhere pretty much applies. I don’t mind the smell, however some people are much more sensitive to it and will find it truly disgusting and will
smell its impregnated smell for months.
While opening the vessel and later on hydrolyzing the thioamide, you should know that H2S is only two times less deadly than HCN. That should be a
consolation, but mind that it is one HELL of a SMELL.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Don't you think you are exaggerating big time. You don't need a hardcore metal autoclave. The pressure stays below 5 bar so you can use simple and
ready made solutions. Search the forum for "pressure vessel"! (Or should I say UTFSE?) |
Wow, you really have an attitude problem. There's no great task in drilling a hole through the valve of a gas tank, using a camping fitting to connect
it to copper tubing and then attaching a ball valve to the end of the copper tubing for most - sorry this is beyond your means. Ill read the threads
your speaking of, but not because YOU told me to.
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
https://sciencemadness.org/talk/viewthread.php?tid=3225#pid3...
'Under stirring and heated at 93-95 C for 3-4 hours. Fluids were evaporated from the mixture, it was allowed to cool, and extracted 3 times with 500
ml hot water. The decolorized solution after standing yielded 26.7 g (97%) white sheets having MP of 157-158 C and BP of 280-290. (Phenylacetamide
C8H9NO)'
Whaddya know? How you like them apples chemistry boy?
[Edited on 31-8-2006 by visitation123]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Sorry for my bad attitude. It was not meant to be as such, but more as a stimulation (or kick) to find some simpler solutions by yourself without me
doing the search for you. Just consider it as a cultural difference. For some reason I’m unable of being pathologically polite. Bear with my
personality like I do or simply ignore my replies.
Anyway, I would certainly not use the vessel you described for this reaction and certainly not only because of the unnecessary fuss of building it.
See Not_important’s reply above for initial information. But to give an additional hint, I can tell you that uncoated steal (interior of a gas tank)
in the presence of polysulfides at 100°C is a “don’t-trust-that-pressure-bomb” kind of thing. In short, corrosion of steal with polysulfides
results in a non-adherent film of iron sulfides. To get a better picture, imagine your gas tank consistently corroding from the inside until the 2-3
bars of pressure blows it apart (makes for nice stinky bomb, now that I think of it). 
Uncoated steal is one of the worst choices for such a corrosive mixtures like ammonium polysulfides.
Hopefully, you will understand why the alternative I offered you is better when you find the relevant threads.
I have not read the translation in the link you provided before and I took it that you meant what you wrote: “the phenylacetamide is extracted with
H2O”. While in the translation it says that hot water is used which when cooled yielded the product as a precipitate. In chemical terminology this
is generally called a recrystallization and not extraction, though the first part (dissolution) is indeed equivalent to an extraction. Nevertheless,
if you consider using 1,5 litters of water for the recrystallization of 27g of product as practical then just do it. I have no problem with that since
I’m not the one who is going to do that. Also, don’t trust that translation 100%. I don’t know if you have noticed it, but there already is an
obvious mistake there: “100 g of the above phenylacetonitrile”. There might be similar ones that can not be identified easily. Note also,
that the yield given (97%) is suspect and can only be explained by the simple fact that what is said to be phenylacetamide is either not well dried or
actually a mixture of phenylacetamide and phenylthioacetamide (and the m.p. the usual bluff).
So I warn you (kindly, this time) again to be careful not to kill yourself with H2S.
I would also suggest you to substitute ethanol with isopropanol as the cosolvent in that procedure. This way you get better solubility of styrene,
lower internal pressure and get faster sulfur consumption. (i-PrOH performed well as a cosolvent in certain other Willgerodt examples).
Regards,
your chemistry boy 
Edit: Gave additional info in order to improve my bad attitude.
[Edited on 31-8-2006 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
visitation123
Harmless

Posts: 34
Registered: 25-8-2006
Member Is Offline
Mood: No Mood
|
|
Your forgiven chemistry boy, you've helped me before whether your aware of it or not.
Isopropanol it may be.
As for the pressure vessel, how would this suffice: a wine bottle fitted with a PVC T fitting, a balloon and a plastic ball valve connected to some
PVC tubing/bubbler submerged in a basin of water, so that when/if the balloon fills pressure can be released with the ball valve and the excess gases
including H2S can be bubbled through the water.
Needless to say, evaporating the fluids is going to stink, so a filtration might be a better option as you and some lit suggests...
|
|
|
| Pages:
1
2
3 |