| Pages:
1
2 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
α-pinene to α-terpineol acetate
The Wilki entry on α-pinene claims that (unspecified) treatment of the latter with glacial acetic acid yields α-terpineol acetate.
If true that would be an addition/rearrangement with no leaving group or by-product(s). Below is NOT an attempt at explaining the reaction mechanism
but rather the diagram shows where the various bits and pieces of the reagents end up (assuming there is such a reaction at all):
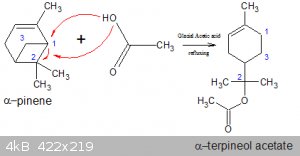
The acidic H would end up on the atom labelled 1, the bond between 1 and 2 would open up and the
acetate group bonded to 2. Atom 3 then ensures the cyclohexene ring remains closed.
But I'm far from understanding electron movements here. I suspect auto-protonation of the GAA may play a part but can't see how.
What's more, I can't find references to this process, re. reagent quantities, temperatures and (presumed?) reflux times and work-up procedures.
Any insights would be most welcome.
[Edited on 6-12-2015 by blogfast25]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Here is a paper that might lend a little bit of insight. It's not much, but maybe it will help.
Attachment: Hydration and acetoxylation of monoterpenes catalyzed by heteropoly acid.pdf (103kB)
This file has been downloaded 1022 times
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Is that product terpenyl vs terpinol? I don't see an alcohol group. The paper from Crowfjord shows terpenyl.
[Edited on 6-12-2015 by Magpie]
[Edited on 6-12-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | Is that product terpenyl vs terpinol? I don't see an alcohol group. The paper from Crowfjord shows terpenyl.
|
The reaction product in my diagram is α-terpinol acetate. There's no alcohol group because the alcohol has been esterified.
In the paper they call the alcohol α-terpineol and the acetate α-terpenyl acetate. Not sure why...
Thanks, Crowfjord.
[Edited on 6-12-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Is this not the same compound:
https://pubchem.ncbi.nlm.nih.gov/compound/alpha-terpinyl_ace...
edit: I see in the synonyms it can be called terpineol.
[Edited on 6-12-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, they appear to be synonymous. Trivial names, eh? 
[Edited on 6-12-2015 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
What a pretty reaction!
Here's my attempt at a possible mechanism:
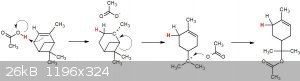
I would think you'd need a much stronger acid to catalyse this, acetic acid is probably too weak to protonate the alkene and get the ball rolling...
[Edited on 6-12-2015 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
What a pretty reaction mechanism!  But is it based on anything? But is it based on anything?
I'll grant you this: the acidic H seems likely to play a part in it. Which makes me wonder aloud whether conc. H2SO4 might be a
catalyst to consider here?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hey I just edited my post to exactly that, yes I think one needs a very strong acid here to generate the carbocations at a good rate.
So then the acetic acid doesn't actually do the protonation in the mechanism, but rather the strong acid catalyst.
Heteropolyacids would work well.
I take it you're wanting to try this one? I just want to know what the product smells like, should be pretty intense 
[Edited on 6-12-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Based on 100% brain fart. It's just me practicing electron pushing, no doubt poorly.
Here's mechanism V2.0 assuming this needs a strong acid catalyst to proceed.
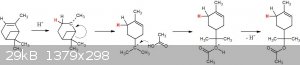
That mechanism reminds me of something...
[Edited on 6-12-2015 by deltaH]

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
H3PW12O40 is the acid recommended in Crowfjord's paper.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hmm... yes, it would help if I read through the thread before posting, sorry, my bad 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Interesting bit from that "Hydration and acetoxylation of monoterpenes catalyzed by heteropoly acid" paper here:
| Quote: | The molar catalytic activities of PW in acetoxylation/hydration
of limonene, -pinene and �-pinene are
approximately 80 times higher than those of H2SO4,
as found from the initial reaction rates. For limonene,
the initial rates with HPA and H2SO4 were found
to be 0.156 and 0.0020 mol l−1 h−1, respectively, at
40◦C, [catalyst] = 0.0060 mol l−1, [limonene] =
0.30 mol l−1 and HOAc/H2O = 90/10. For -pinene,
they were 0.270 and 0.0040 mol l−1 h−1 at 25◦C and
for �-pinene 0.358 and 0.0050 mol l−1 h−1 at 15◦C
and otherwise the same conditions. This is in accordance
with the relative acid strengths of PW and
H2SO4 in acetic acid (pK1 4.8 and 7.0, respectively
[29]). |
Didn't realise just how strong these poly acids were...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yes, I knew that  , I even prepared one a VERY long time ago, it was one of the
molybdenum, vanadium, phosphate ones that's used for organic oxidations with O2. I forget what it was called, it had a catchy acronym for a name and I
think it was an ammonium salt. Anyway, not relevant to this thread and I don't remember much more about it than that unfortunately. , I even prepared one a VERY long time ago, it was one of the
molybdenum, vanadium, phosphate ones that's used for organic oxidations with O2. I forget what it was called, it had a catchy acronym for a name and I
think it was an ammonium salt. Anyway, not relevant to this thread and I don't remember much more about it than that unfortunately. 
EDIT: Ah found it, it was called NPMoV... not really that catchy of an acronym now that I see it again. I also found the paper I was working from and
the application I was using it for, top of page 22, FYI. It was cool chemistry.
Attachment: NPMoV.pdf (325kB)
This file has been downloaded 635 times
[Edited on 6-12-2015 by deltaH]
|
|
|
AvBaeyer
National Hazard
   
Posts: 655
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
There is a fair amount of information on the conversion of a-pinene to a-terpineol acetate if you do the search in Google scholar. Bottom line is
that, as already pointed out above, a strong acid catalyst is required to carry out the reaction. A quick search gave the attached paper as an
example.
AvB
Attachment: Conversion of a-pinene to terpinyl acetate over zeolitespdf.pdf (361kB)
This file has been downloaded 624 times
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Looks like sulfuric acid is used to prepare the terpenol in a two-step process, followed by esterification with acetic anhydride, so I'm guessing
sulfuric acid + acetic in a one-step reaction doesn't work well/is messy. Guess you need either a heteropoly acid or zeolite catalyst then.
Heteropolyacids are very easy to prepare if you have the reagents. Also a useful catalyst to have in the 'toolbox'.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Here are my relevant papers on production of alpha-terpineol. I also recall a prep using trichloroacetic acid in place of the trifluoroacetic.
Attachment: terpineol via F3CCOOH and limonene.pdf (33kB)
This file has been downloaded 473 times
Attachment: Hydration of turpentine to terpineol.pdf (60kB)
This file has been downloaded 505 times
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Nice! I wonder if the local dentist is willing to part with a little, they use it to etch teeth prior to applying filling epoxies AFAIK
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thank you UC235 and AvBaeyer.
I'm still inclined to go with the GAA+poly phosphotungstic acid after reading your papers, although other opinions will be considered.
Poly phosphotungstic acid is expensive to buy but relatively easy to prepare, although I've yet to find a free (non-paywalled)
preparation procedure for it. As deltaH mentioned, it's a real good thing to have in the larder too.
Years ago I attempted to prepare α-terpineol from natural turpentine, H2SO4 and acetone and all I got (at RT) was an ever darkening mass over a few
days, going basically black over a week. Frustrating...
[Edited on 7-12-2015 by blogfast25]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Quote: Originally posted by blogfast25  |
I'm still inclined to go with the GAA+poly phosphotungstic acid after reading your papers, although other opinions will be considered.
Poly phosphotungstic acid is expensive to buy but relatively easy to prepare, although I've yet to find a free (non-paywalled)
preparation procedure for it. As deltaH mentioned, it's a real good thing to have in the larder too.
[Edited on 7-12-2015 by blogfast25] |
Do you have the reference information for these preparations, blogfast? There are plenty of us here willing to help with access to such things.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Searching for terpinyl acetate brought up Shitao Yu as one of the cited researchers on a paid-for site.
Searching "Shitao Yu terpinyl acetate" found a free version.
It wasn't a pdf file extension, so here is the renamed file :
Attachment: terpinylacetate.pdf (609kB)
This file has been downloaded 808 times
[Edited on 7-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Crowfjord  | [
Do you have the reference information for these preparations, blogfast? There are plenty of us here willing to help with access to such things.
|
I've found some old preps in Brauer's book in the SM library, pages 1720 (sodium salt) and 1700 (Dreschel's method to obtain the free acid). But I'm
looking for something more modern like:
http://onlinelibrary.wiley.com/doi/10.1002/jctb.5000690906/a...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Searching for terpinyl acetate brought up Shitao Yu as one of the cited researchers on a paid-for site.
Searching "Shitao Yu terpinyl acetate" found a free version.
It wasn't a pdf file extension, so here is the renamed file :
[Edited on 7-12-2015 by aga] |
I'll have to it take to the 'Canton Dragon', around the corner... I'll get some
chow mein to go as well. I'll get some
chow mein to go as well.
[Edited on 7-12-2015 by blogfast25]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
I don't have access to the full article, but luckily CA/Scifinder gives an abbreviated preparation:
| Quote: |
Na phosphotungstate (I) is prepd. by adding 200 cc. concd. HCl to a boiling soln. of 250 g. Na tungstate (II) and 37.5 g. Na2HPO4 in 300 cc. H2O.
After chilling, the ppt. is filtered and dried. The air-dried crude product (180 g.) is recrystd. from H2O (80 cc.), yield 135 g. of a product
having a variable amt. of water of crystn. The mother liquor from the recrystn. can be reused. All mother liquors in the process can be treated
with BaCl2 crystals to obtain Ba phosphotungstate (III). III is prepd. by adding 250 g. BaCl2.2H2O in 800 cc. boiling H2O 330 g. Na tungstate in 1
l. boiling H2O. After cooling, the ppt. is filtered, washed with hot H2O and dried at 90°, yield 398 g. of Ba tungstate (IV), sparingly sol. in
H2O. 3.5 cc. of 86° H3PO4 is added to 160 g. IV suspended in 250 cc. boiling H2O. Concd. HCl (100 cc.) is added and the mixt. boiled for 2 hrs.
Completion of the reaction can be detd. microscopically since the cryst. form of the suspension changes. The yield of filtered, washed, and
air-dried III is 120 g. To prep. III from I, a soln. of I (130 g.) in boiling water (300 cc.) is added to a soln. of BaCl2.2H2O (40 g.) in boiling
water. Upon concn. to 350 cc. and cooling, III crystallizes out. The yield of washed and air-dried product is 127 g. To prep. phosphotungstic
(V) acid from IV, 240 g. IV is suspended in 200 cc. boiling water and treated with 5 cc. 86% H3PO4, then with a mixt. of 33.5 cc. concd. H2SO4 and 100
cc. water with agitation and boiling. After 2 hrs. the BaSO4 is filtered off while hot. The filtrate is concd. to dryness under reduced pressure.
The yield of V is 160 g. It can be purified with charcoal. The soly. in water at 20° is approx. 5 g. per cc. To prepare V from III, 200 g. of
the Ba salt is suspended in a l. of boiling water and a mixt. of concd. 5.4 cc. H2SO4 and 50 cc. water is slowly added with agitation and boiling.
The boiling is continued for 1 hr., charcoal is added, the BaSO4 is filtered off, and the filtrate evapd. to dryness at reduced pressure. The
yield is 180 g.
|
A similar preparation from Zhurnal Obshchei Khimii (1955), Volume25 Pages 2388-91:
| Quote: |
To prep. H7P(W2O7)6], mix 1.3 g. BaWO4 (prepd. by pptg. Na2WO4 with BaCl2) with 2.031 l. of boiling H2O, add 28 ml. H3PO4 (87.9%) to the suspension,
boil for 15 min., add 812.5 ml. of concd. HCl, and stir for ∼2 hrs. Dissolve the Ba3H8[P(W2O7)6]2 in boiling H2O (1 kg.:5 l.) add 27 ml. of concd.
H2SO4, boil with stirring for 1 hr., and filter off the BaSO4. Evap. the filtrate to dryness in vacuo. The yield is 76%.
To prep. H7P(W2O7)6], mix 1.3 g. BaWO4 (prepd. by pptg. Na2WO4 with BaCl2) with 2.031 l. of boiling H2O, add 28 ml. H3PO4 (87.9%) to the suspension,
boil for 15 min., add 812.5 ml. of concd. HCl, and stir for ∼2 hrs. Dissolve the Ba3H8[P(W2O7)6]2 in boiling H2O (1 kg.:5 l.) add 27 ml. of concd.
H2SO4, boil with stirring for 1 hr., and filter off the BaSO4. Evap. the filtrate to dryness in vacuo. The yield is 76%
|
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Once you have menu item numbers 16, 23, 47 and of course 54 (two portions) safely back home, scroll down past the Chinese 1st page to get to the
synthesis in English.
It is short, so you'll be wanting more to eat by the time you finish reading it.
Edit:
Astonishing that there are still a handful of people who still cannot read Canton Chinese ...
[Edited on 7-12-2015 by aga]
|
|
|
| Pages:
1
2 |