bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Preparation of dichloroethylene from ethanol?!
Hi everyone!!
I would like to prepare dichloroethylene from ethanol.
I have a few questions about :
1.At what temperature can I dehydrate ethanol?
2.Can sulfuric acid used as a catalyst turn to a gas during this process?
3.In what conditions can I complete the second step using chlorine, ethylene, & iron (III) chloride?
4.I found the paragraph below on wikipedia, it talks about an alternative method. Can anyone explain that?
"In principle, it can be prepared by the chlorination of ethane and, less directly, from ethanol."
https://en.m.wikipedia.org/wiki/1,2-Dichloroethane
[Edited on 1-12-2015 by bluamine]
[Edited on 2-12-2015 by bluamine]
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Ethanol cannot be dehydrated with heat. Water boils at a higher temperature than ethanol. A hygroscopic salt, typically potassium carbonate, is used
to separate water from the alcohol. As for the reaction, I know very little organic.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Detonationology  |
Ethanol cannot be dehydrated with heat. Water boils at a higher temperature than ethanol. A hygroscopic salt, typically potassium carbonate, is used
to separate water from the alcohol. As for the reaction, I know very little organic. |
Hi dear!
I was not talking about drying ethanol, I know how to do that using sodium sulfate, copper sulfate, or even calcium oxide. I was talking about making
ethylene as a first step of dichloroethylene synthesis process. I know heat doesn't do that, so I must use a catalyst (sulfuric acid) as it is
mentioned above.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Adding ethanol dropwise to sulfuric acid at or above 160°C, though I would increase that to about 175°C to ensure complete dehydration and minimize
the formation of ether. This reaction will be a pain, hands down. Any reaction which involves two gases reacting with each other is enough trouble
already, but that's using cylinders of compressed gas to have easy, precise control over the flow rate. Generating both the chlorine and the ethylene
chemically will make it nearly impossible to control the flow rates precisely enough, and the reaction will thus be essentially undoable in an amateur
lab.
If you want dichloroethane, I would use the reaction of Lucas' Reagent with ethylene glycol, which can be obtained by the permanganate oxidation of
ethylene if absolutely necessary. Distilling antifreeze will be a much simpler endeavor however, if it is available.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Ah, (facepalm) I see now! As long as the catalyst stays hot, the dehydration should work without any heating of the ethanol. If the ethanol boils,
you will risk not completely dehydrating the ethanol, and the lost ethanol vapor would likely H bond with the water upon contact.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | Adding ethanol dropwise to sulfuric acid at or above 160°C, though I would increase that to about 175°C to ensure complete dehydration and minimize
the formation of ether. This reaction will be a pain, hands down. Any reaction which involves two gases reacting with each other is enough trouble
already, but that's using cylinders of compressed gas to have easy, precise control over the flow rate. Generating both the chlorine and the ethylene
chemically will make it nearly impossible to control the flow rates precisely enough, and the reaction will thus be essentially undoable in an amateur
lab.
If you want dichloroethane, I would use the reaction of Lucas' Reagent with ethylene glycol, which can be obtained by the permanganate oxidation of
ethylene if absolutely necessary. Distilling antifreeze will be a much simpler endeavor however, if it is available. |
Well i was looking for ethylene glycol manufactur, because i was not sure about other chemicals existing in a antifreeze. How simple can distillation
process be? Any specific conditions? & after it is done, must I use any other methods to separate any probably remaining impurities?
[Edited on 1-12-2015 by bluamine]
[Edited on 2-12-2015 by bluamine]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Distillation of an ethylene glycol antifreeze is actually rather straightforward. A simple distillation suffices to separates the ethylene glycol
from the various dyes and other crap in the antifreeze. Use a fractionating column if you wish to get purer ethylene glycol, but it isn't strictly
necessary. A vacuum will also help to lower the necessary distillation temperature, but again it is not strictly necessary.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
I read first about another method based on reacting ethylene with hypochlorous acid to produce 2chloroethanol, then reacting this last with sodium
hydroxide to get ethylene oxide, & lastly reacting ethylene oxide with water. It is clear that this process is very complicated, in addition, I
read that it can cause some impurities in the final products.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Dear gdflp
What about the other method I quoted from Wikipedia (ethanol chlorination)? (Only as an alternative process)
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I don't see how ethanol chlorination can lead to dichloroethane. Chlorination of ethane could proceed via a radical mechanism, but you again run into
the issues of a reaction involving two gases. In addition, regioselectivity is going to be absolutely abysmal; the process is only viable on a
massive industrial scale, where they have access to very efficient columns and the economy of scale.
Ethylene oxide is quite nasty, not trying to be condescending, but you don't sound equipped to handle it. And that method is very tedious for
something as simple as ethylene glycol, it's simply not worth it.
What is your end goal here, dichloroethane or ethylene glycol? And if it is the former, do you specifically need that solvent, or can it replaced
with another chlorinated solvent?
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | I don't see how ethanol chlorination can lead to dichloroethane. Chlorination of ethane could proceed via a radical mechanism, but you again run into
the issues of a reaction involving two gases. In addition, regioselectivity is going to be absolutely abysmal; the process is only viable on a
massive industrial scale, where they have access to very efficient columns and the economy of scale.
Ethylene oxide is quite nasty, not trying to be condescending, but you don't sound equipped to handle it. And that method is very tedious for
something as simple as ethylene glycol, it's simply not worth it.
What is your end goal here, dichloroethane or ethylene glycol? And if it is the former, do you specifically need that solvent, or can it replaced
with another chlorinated solvent? |
I do know some properties of ethylene oxide this us why I did not think I can do that process. I just mentioned it.
My final goal is ethylene glycol, no doubt that I can use other chlorinated solvents, otherwise, I would use chloroform instead, which is available
for me, & I can even produce it if I needed some relatively big amounts, by reacting sodium hypochlorite & acetone.
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
By dichloroethylene do you mean 1,2-Dichloroethane (Ethylene dichloride) or 1,2-Dichloroethene (The alkene instead of the alkane)?
> 1. At what temperature can I dehydrate ethanol?
Ethanol can be dehydrated to Ethene (Ethylene) by heating it at above 150°C with concentrated sulfuric acid. I don't know the optimum quantity of
H2SO4 catalyst but if you don't use enough H2SO4 you won't be able to get your reaction mixture to 150°C without the ethanol boiling off. I know this
reaction can be easily done because I have often synthesized lots of Ethylene gas when attempting to synthesize diethyl ether but accidentally letting
the reaction temp get too high. If you can't easily find any written procedures for this reaction online, just adapt an ether prep but run the
reaction hotter. (This reaction is exceedingly dangerous, and could just spontaneous explode unless you are incredibly cautious and watch the reaction
temps like a hawk)
> 2.Can sulfuric acid used as a catalyst turn to a gas during this process?
conc. H2SO4 boils at 338°C, so you shouldn't worry about it boiling off at the temps you should be working at. conc. Phosphoric acid is also a good
dehydrating agent, with the added benefit of it being non-oxidising. Thus reducing potentially dangerous side reactions. If you can find any good
preps for ethanol dehydration with H3PO4 I think that would be the way to go.
>3. In what conditions can I complete the second step using chlorine, ethylene, & iron (III) chloride?
Without opening Wikipedia to actually read up on it, I'd guess this is an industrial reaction. They probably have large reactors in china running at
very high temps, very high pressures and a fancy catalyst supports. Reactions between two gasses is always tricky in a lab environment, never mind in
the home lab. An alternative approach might be bubbling the generated ethylene gas through a solution of bromine in a solvent like n-hexane, or
through a dilute aqueous solution of KMnO4 (look up Baeyer's reagent) to obtain 1,2-Dibromoethane (Ethylene dibromide) or Ethane-1,2-diol (Ethylene
glycol) respectively. These compounds can then easily be converted 1,2-Dichloroethane (Ethylene dichloride).
>4. I found the paragraph below on wikipedia, it talks about an alternative method. Can anyone explain that?
They're probably referring to free-radical substitution reactions. This is another industrial reaction and it creates several different poly
chlorinated by-products. This can be done in a home lab, but honestly its gonna' be much more trouble than it's worth.
If you have your heart set on making 1,2-Dichloroethene from Ethanol I would use the following steps:
1. Strongly heat a mixture of conc. Phosphoric acid and Ethanol in a flask with a gas take-off adapter.
2. Lead the gas into a flask containing an ice-cold alkaline solution of KMnO4 until no more purplish-pink color is observed.
3. Filter off the brown Manganese dioxide precipitate.
4. Fractionally distil the filtrate and isolate the ethylene glycol fraction
5. Reflux the ethylene glycol with an excess of Zinc chloride and conc. Hydrochloric acid
6. Distil out the lower boiling 1,2-Dichloroethane from the reaction mixture.
7. Dehydrohalogenate with an alcoholic solution of KOH to create Vinyl chloride gas.
10. Lead the gas into a flask containing bromine in a solvent such a n-Hexane until the solution turns colorless.
11. Isolate the 1,2-Dibromo-1-chloroethane and react with sodium chloride in DMF to convert it to 1,1,2-Trichloroethane.
12. Dehydrohalogenate with an alcoholic solution of KOH to create 1,2-Dichloroethene
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NitreRat  | By dichloroethylene do you mean 1,2-Dichloroethane (Ethylene dichloride) or 1,2-Dichloroethene (The alkene instead of the alkane)?
> 1. At what temperature can I dehydrate ethanol?
Ethanol can be dehydrated to Ethene (Ethylene) by heating it at above 150°C with concentrated sulfuric acid. I don't know the optimum quantity of
H2SO4 catalyst but if you don't use enough H2SO4 you won't be able to get your reaction mixture to 150°C without the ethanol boiling off. I know this
reaction can be easily done because I have often synthesized lots of Ethylene gas when attempting to synthesize diethyl ether but accidentally letting
the reaction temp get too high. If you can't easily find any written procedures for this reaction online, just adapt an ether prep but run the
reaction hotter. (This reaction is exceedingly dangerous, and could just spontaneous explode unless you are incredibly cautious and watch the reaction
temps like a hawk)
> 2.Can sulfuric acid used as a catalyst turn to a gas during this process?
conc. H2SO4 boils at 338°C, so you shouldn't worry about it boiling off at the temps you should be working at. conc. Phosphoric acid is also a good
dehydrating agent, with the added benefit of it being non-oxidising. Thus reducing potentially dangerous side reactions. If you can find any good
preps for ethanol dehydration with H3PO4 I think that would be the way to go.
>3. In what conditions can I complete the second step using chlorine, ethylene, & iron (III) chloride?
Without opening Wikipedia to actually read up on it, I'd guess this is an industrial reaction. They probably have large reactors in china running at
very high temps, very high pressures and a fancy catalyst supports. Reactions between two gasses is always tricky in a lab environment, never mind in
the home lab. An alternative approach might be bubbling the generated ethylene gas through a solution of bromine in a solvent like n-hexane, or
through a dilute aqueous solution of KMnO4 (look up Baeyer's reagent) to obtain 1,2-Dibromoethane (Ethylene dibromide) or Ethane-1,2-diol (Ethylene
glycol) respectively. These compounds can then easily be converted 1,2-Dichloroethane (Ethylene dichloride).
>4. I found the paragraph below on wikipedia, it talks about an alternative method. Can anyone explain that?
They're probably referring to free-radical substitution reactions. This is another industrial reaction and it creates several different poly
chlorinated by-products. This can be done in a home lab, but honestly its gonna' be much more trouble than it's worth.
If you have your heart set on making 1,2-Dichloroethene from Ethanol I would use the following steps:
1. Strongly heat a mixture of conc. Phosphoric acid and Ethanol in a flask with a gas take-off adapter.
2. Lead the gas into a flask containing an ice-cold alkaline solution of KMnO4 until no more purplish-pink color is observed.
3. Filter off the brown Manganese dioxide precipitate.
4. Fractionally distil the filtrate and isolate the ethylene glycol fraction
5. Reflux the ethylene glycol with an excess of Zinc chloride and conc. Hydrochloric acid
6. Distil out the lower boiling 1,2-Dichloroethane from the reaction mixture.
7. Dehydrohalogenate with an alcoholic solution of KOH to create Vinyl chloride gas.
10. Lead the gas into a flask containing bromine in a solvent such a n-Hexane until the solution turns colorless.
11. Isolate the 1,2-Dibromo-1-chloroethane and react with sodium chloride in DMF to convert it to 1,1,2-Trichloroethane.
12. Dehydrohalogenate with an alcoholic solution of KOH to create 1,2-Dichloroethene
|
Well thank you for all thèse informations. Honestly it seems you are a genius. I wish thé best for you.
I am not sure about what must I use (alkane or alkine), I just read about this reaction on the link below:
http://www.ucc.ie/academic/chem/dolchem/html/comp/glycol.htm...
It is mentioned on that page that this reaction can be done in a lab, this is why I did prefered to use it. I have access to university's labs, so
even if I couldn't do it at home, I would do it at university if the information mentioned there was correct.
I just made a small amount of hydrobromic acid, using seawater, it was really hard, & the harder is to reduce it to bromine though I don't need to
 (my final purpose is to obtain pure ethylene glycol, as mentioned above) (my final purpose is to obtain pure ethylene glycol, as mentioned above)
I can buy phosphate easily & it is relatively cheap. So I can use it to make phosphoric acid. Permanganate solution can be bought easily, but it
is really diluted (I don't have any idea about another commercial solution than the one existing in pharmacies), so i f I need some bigger amounts I
can't buy from there (I am just supposing), I can look for an easy way to make it using a potassium salt, & manganese dioxide.
[Edited on 1-12-2015 by bluamine]
[Edited on 1-12-2015 by bluamine]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by NitreRat  | | Phosphoric acid is also a good dehydrating agent, with the added benefit of it being non-oxidising. Thus reducing potentially dangerous side
reactions. If you can find any good preps for ethanol dehydration with H3PO4 I think that would be the way to go. |
I wouldn't use phosphoric acid in a glass flask at 150°C+. The acid's etching effect on glass is not noticeable at room
temperature, but it becomes pronounced at high temperatures. A glass flask full of phosphoric acid at those temperatures could be completely
destroyed, and 160°C phosphoric acid is not something which I would want spilling over a hot plate.
Quote: Originally posted by NitreRat  |
7. Dehydrohalogenate with an alcoholic solution of KOH to create Vinyl chloride gas.
12. Dehydrohalogenate with an alcoholic solution of KOH to create 1,2-Dichloroethene
|
Dehydrohalogenations are not very easy to do that precisely. There would be significant losses due to side reactions if you tried to get a haloalkene
from a dihaloalkane.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by bluamine  |
It is mentioned on that page that this reaction can be done in a lab, this is why I did prefered to use it. I have access to university's labs, so
even if I couldn't do it at home, I would do it at university if the information mentioned there was correct.
I just made a small amount of hydrobromic acid, using seawater, it was really hard, & the harder is to reduce it to bromine though I don't need to
 (my final purpose is to obtain pure ethylene glycol, as mentioned above) (my final purpose is to obtain pure ethylene glycol, as mentioned above)
I can buy phosphate easily & it is relatively cheap. So I can use it to make phosphoric acid. Permanganate solution can be bought easily, but it
is really diluted (I don't have any idea about another commercial solution than the one existing in pharmacies), so i f I need some bigger amounts I
can't buy from there (I am just supposing), I can look for an easy way to make it using a potassium salt, & manganese dioxide.
|
If you're goal is to get ethylene glycol, then the oxidation of ethylene with potassium permanganate is the best, and most direct, option. I would
boil down the dilute permanganate solution to concentrate it as much as possible, then simply bubble ethylene through it. From there, neutralize
excess permanganate in the solution with very small amounts of sodium metabisulfite or another similar reducing agent, be careful not to add too much
and redissolve some of the manganese dioxide as manganous ions. Finally, filter off the manganese dioxide sludge, and fractionally distill the mother
liquor to get relatively pure ethylene glycol. Be aware that you won't get very much with this process, theoretical yield is ~30ml ethylene glycol
per liter of concentrated aqueous potassium permanganate.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | | I wouldn't use phosphoric acid in a glass flask at 150°C+. The acid's etching effect on glass is not noticeable at room temperature, but it becomes
pronounced at high temperatures. A glass flask full of phosphoric acid at those temperatures could be completely destroyed, and 160°C phosphoric
acid is not something which I would want spilling over a hot plate. |
I will use some alumina ceramic instead, does is resist phosphoric acid?
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  |
Quote: Originally posted by NitreRat  |
7. Dehydrohalogenate with an alcoholic solution of KOH to create Vinyl chloride gas.
12. Dehydrohalogenate with an alcoholic solution of KOH to create 1,2-Dichloroethene
|
Dehydrohalogenations are not very easy to do that precisely. There would be significant losses due to side reactions if you tried to get a haloalkene
from a dihaloalkane. |
Yes, I was aware that these steps were a bit assumptious. If nothing else, I was just trying to highlight how frustrating and low yielding the
processes of ethanol to dichloroethene would be. In a real lab I would probably use Potassium tert-butoxide in an ether solvent and control the
stoichiometry as best I could for the dehydrohalogenations, but in a real lab I would probably chose to start with acetylene instead of ethanol.
CaC2(s) + 2 H2O(l) → C2H2(g) + Ca(OH)2(s)
C2H2(g) + Br2(hexane) → C2H2Br2(l) + (over-brominated product by-product, but tetrabromoethane might also be useful
for something so I wouldn't throw it away)
C2H2Br2(DMF) + 2NaCl(DMF) → C2H2Cl2(l) + 2NaBr(DMF)
[Edited on 12/2/2015 by NitreRat]
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | | I wouldn't use phosphoric acid in a glass flask at 150°C+. The acid's etching effect on glass is not noticeable at room temperature, but it becomes
pronounced at high temperatures. A glass flask full of phosphoric acid at those temperatures could be completely destroyed, and 160°C phosphoric
acid is not something which I would want spilling over a hot plate. |
Thanks, for the info. I completely forgot about it's corrosive effect on glassware. Molten hydroxides, Hydrofluoric acid and hot Phosphoric acid are
the major glass killers, if my memory serve me well. I've even seen hot solutions of NaOH in glycerol eat through borosilicate. The reason I initially
suggested H3PO4 is because I believe that's how the reaction is done industrially (or at least hydration of ethylene to ethanol). Hot activated
alumina, Phosphorus pentoxide(if you're brave enough) and Benzenesulfonic acid(?) might be potential alternatives. H2SO4 is still probably the best
bet at this scale though. There must be a tonne of available literature out there for the initial dehydration reaction.
[Edited on 12/2/2015 by NitreRat]
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Only if your alumina ceramic round bottom flask is compatible with your other ground glass joints. 
I suppose you could get away with doing that reaction in a tin can, with a copper condenser welded on the top, like some of the guys did when making
decarboxylating sodium benzoate to benzene. Nile Red's video on it even looked quite
professional.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
This is noticeable bro lol
Quote: Originally posted by NitreRat  | | I suppose you could get away with doing that reaction in a tin can, with a copper condenser welded on the top, like some of the guys did when making
decarboxylating sodium benzoate to benzene. Nile Red's video on it even looked quite
professional. |
but tin melting point is too low.. If I overheat it melts..
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  |
If you're goal is to get ethylene glycol, then the oxidation of ethylene with potassium permanganate is the best, and most direct, option. I would
boil down the dilute permanganate solution to concentrate it as much as possible, then simply bubble ethylene through it. From there, neutralize
excess permanganate in the solution with very small amounts of sodium metabisulfite or another similar reducing agent, be careful not to add too much
and redissolve some of the manganese dioxide as manganous ions. Finally, filter off the manganese dioxide sludge, and fractionally distill the mother
liquor to get relatively pure ethylene glycol. Be aware that you won't get very much with this process, theoretical yield is ~30ml ethylene glycol
per liter of concentrated aqueous potassium permanganate. |
What is exactly the temperature condition? How much sulfuric acid & ethanol should I use to obtain this amount (30 ml) of ethylene glycol?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Tin cans aren't actually tin, they're usually steel.
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
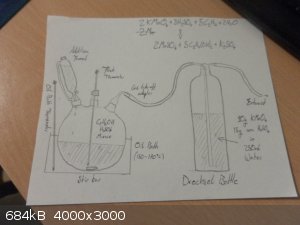
I drew a diagram of the sort of set-up you might employ for this reaction. Unfortunately, unless I miscalculated, the stoichiometry doesn't work out
very nicely and 30ml of ethylene glycol corresponds to about 34g of KMnO4 and a similar amount of H2SO4. Which would
be a very concentrated solution in 250ml of water. So you would probably get a lot of over oxidation. However in my equation I assumed that the
MnO4- was being reduced all the way down to Mn2+. The nice thing about this way of performing the reaction is that you get a
nice color change, from dark purple to almost colorless pale pink in the drechsel bottle. Which indicates when the reaction is complete.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NitreRat  |
I drew a diagram of the sort of set-up you might employ for this reaction. Unfortunately, unless I miscalculated, the stoichiometry doesn't work out
very nicely and 30ml of ethylene glycol corresponds to about 34g of KMnO4 and a similar amount of H2SO4. Which would
be a very concentrated solution in 250ml of water. So you would probably get a lot of over oxidation. However in my equation I assumed that the
MnO4- was being reduced all the way down to Mn2+. The nice thing about this way of performing the reaction is that you get a
nice color change, from dark purple to almost colorless pale pink in the drechsel bottle. Which indicates when the reaction is complete.
|
Thank you bro.I have several questions here:
1.What about sulfuric acid & ethanol amounts in the flask?
2.Why did you add the additional funnel?
3.What are the contents of the oil bath?
4.If the permanganate solution should be this concentrated, it should not be really cold.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
But I don't think that's true in my country. They are not that hard, i can easily crush one of them with a small hammer
|
|
|