ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Is it Co2O3?
Here's an experiment with somewhat puzzling (for me) results.
I was trying to make potassium percobaltate, just for fun, the same way as potassium ferrate is made: by oxidizing cobalt hydroxide with a very
alkaline solution of KOH and NaClO.
It gave a suspension of black powder that does not dissolve in water, neither does it react with acids (I tried conc sulfuric and dilute
hydrochloric). I assume that this is Co2O3.
How do I check if the black powder really is cobalt (III) oxide? And if it truly is, how do I convert it to potassium percobaltate?
Addendum: the powder reacts with dilute hydrogen peroxide, evolving oxygen. I'm under impression that it is not expended, it's a MnO2 type
catalysis. And in general this powder greatly resembles MnO2.
[Edited on 7-9-2015 by ave369]
Smells like ammonia....
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It probably is Co2O3.
Try fusing it with KNO3.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
When it dries, I'll try.
Smells like ammonia....
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
With reference to the following Pourbaix diagrams (1)(2).
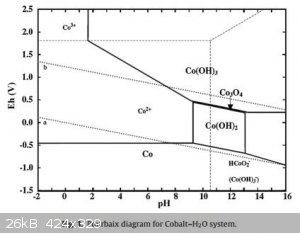 
Co(OH)3 appears to be the speciation under the conditions stated.
Then again, Co(OH)2 might have been oxidised to Co3O4 and not further because of kinetic reasons.
You might want to compare starting not with the hydroxide, but a cobalt salt added to the solution of alkaline hypochlorite. Probably more likely to
make high oxidation states.
References:
(1) E.M. Garcia et al. Journal of Power Sources 185 (2008) 549–553.
(2) Marie Radepont et al. J. Anal. At. Spectrom. 30 (2015) 599-612.
[Edited on 7-9-2015 by deltaH]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
If it's Co(OH)3, will it result in Co2O3 after drying up and calcination? I'm still going to give fusing with KNO3 a try.
Quote: Originally posted by deltaH  |
You might want to compare starting not with the hydroxide, but a cobalt salt added to the solution of alkaline hypochlorite. Probably more likely to
make high oxidation states.
[Edited on 7-9-2015 by deltaH] |
Just tried exactly that (tried cobalt (II) chloride). Got more of the same black Co(III) compound.
[Edited on 7-9-2015 by ave369]
Smells like ammonia....
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Co(OH)3 is above the O2 line on the diagram, so indeed upon drying and calcination I would expect it to decompose to Co3O4 by liberation of oxygen and
water based on the thermodynamics. "Co2O3" appears to be somewhat contentious. As I see it, it would require that Co(OH)3 be dehydrated under such
conditions that the kinetics for it liberating oxygen is slow, so calcining is out, you'd almost certainly make Co3O4.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Okay, then I melt it with KNO3 without calcining.
Smells like ammonia....
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I'd melt it with KOH, it's already oxidised to 3+, you need to preserve it with alkalinity. That way you might drive off water and form the potassium
salt while preventing it from liberating oxygen by being very alkaline throughout, i.e. try to form KCoO2:
Co(OH)3(s) + KOH(l) + heat => KCoO2(s) + 2H2O(g)
I'd start with the moist Co(OH)3, not even dry it, just suction the excess water off at the filter.
You could then try fusing the KCoO2 with KNO3 (maybe even with some KOH for stability) if you want to aim for higher oxidation states, at least you've
given it the best chance to survive.
[Edited on 7-9-2015 by deltaH]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Okay, I'll try that. However, my Black Cobalt (III) Powder is already dry. If this synthesis fails, I'll try to make the Black Cobalt (III) Powder
again (though my stock of CoCl2 is fairly limited).
BTW, the Black Powder wasn't thoroughly washed. It means, it still has remnants of KOH on it. So maybe it won't release oxygen overnight.
[Edited on 7-9-2015 by ave369]
Smells like ammonia....
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
If prepared in an aqueous environment you have probably prepared CoO(OH). This compound occurs naturally as heterogenite and is fairly wide spread in
small amounts. It is insoluble in most acid but is easily rendered soluble by adding a little H2O2 when it even dissolves in acetic acid. I am not
aware of any acidic properties of this compound or of any higher valceny oxi-compound.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have the impression that you made Co3O4. I have some of this powder as well. It is black like soot and does not dissolve in acids and catalytically
decomposes H2O2. It is a mixed +2/+3 oxide of cobalt. I read that Co2O3 is much more reactive. It should dissolve in conc. HCl, giving a blue solution
of the tetrachlorocobalt(II)-complex and chlorine gas. Co3O4 only dissolves in hot conc. HCl very very slowly and it does not dissolve at all in hot
moderately concentrated HNO3 or H2SO4.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
I'll try to prepare some concentrated HCl today and check it out. I'll also try the acid/H2O2 mixture.
Did the experiments.
It does indeed dissolve in a mixture of acid (any) and hydrogen peroxide.
It also reacts with conc. hydrochloric at room temperature. Chlorine evolves, and there is no hint of blue in the solution, it is rather
pinkish-brown. Can't say the concentation of hydrochloric acid, it is homemade, I think it's something like 20%. It fumed noticeably when warm,
somewhat less when cold.
Melting with KNO3 and KOH failed to produce any new substance. I only fouled the crucible.
[Edited on 8-9-2015 by ave369]
Smells like ammonia....
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Maybe try melting it with KClO3?
Smells like ammonia....
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per Atomistry.com on Co(OH)3, link: http://cobalt.atomistry.com/hydrated_cobaltic_oxide.html, to quote:
"Hydrated Cobaltic Oxide, Co(OH)3 or Co2O3.3H2O, is obtained in a more or less complete state of hydration when an alkaline oxidiser, such as, for
example, an alkaline solution of ammonium persulphate, or of sodium hypochlorite, is added to a cobalt salt. The precipitate varies in composition
according to the conditions obtaining at the moment. When an alkaline solution of cobalt sulphate is electrolysed the oxide is obtained in at least
two stages of hydration, namely, the dihydrate, Co2O3.2H2O, and the trihydrate, Co2)O3.3H2O.
As obtained by the foregoing methods, hydrated cobaltic oxide is a blackish brown precipitate which dissolves in aqueous hydrogen chloride evolving
chlorine, cobaltous chloride passing into solution. The oxide thus behaves like a peroxide. "
With well-cooled acids, brownish solutions are obtained which are presumed to contain cobaltic salts."
Depending on conditions, a form of hydrated cobaltic oxide (Co2O3.xH2O) is my best guess.
[Edited on 8-9-2015 by AJKOER]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
What crucible did you use?
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
A porcelain crucible. 25 ml, with a lid. Heated by a Korean butane burner.
[Edited on 8-9-2015 by ave369]
Smells like ammonia....
|
|
|