| Pages:
1
2
3
..
5 |
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Chemistry of Terephthalic acid
The recovery of terephthalic acid from PET bottles is now well described elsewhere on this forum and the topic of what can be done with the resulting
acid has been broached occasionally in various threads. Unfortunately, these threads usually take the form “can I make xxxx from PET bottles?”
rather than looking at what can reasonably be prepared from the free acid.
Has anyone out there ever prepared any derivatives from terephthalic acid and is prepared to share their results with us?
I recently ran a series of experiments on the alkali hydrolysis of PET resin from water bottles and now have roughly 200g of terephthalic acid and
sodium terephthalate. By carrying out the hydrolysis in batches of 50-60g of PET at a time and recycling the liquor into the next batch I managed to
achieve recoveries of >95% of a high quality product. The preparation of solid, crystalline di-sodium terephthalate proved tricky since it appears
that the solubility/temperature gradient is almost flat and so a saturated solution of the sodium salt does not crystallise at all. The best method
was found to be to evaporate the sodium salt solution on a water bath until a slurry of sodium terephthalate crystals had formed and filter.
What next?
Having prepared the acid and sodium salt I decided to investigate its chemistry. I have not yet had chance to investigate it’s chemistry with
practical experiments but in general it appears that terephthalic acid is very stable and not very reactive and direct substitution is difficult and
limited. After a moderately extensive search through the literature I came to the conclusion that there are 6 routes to derivatives of terephthalic
acid that are reasonably achievable to amateurs though not necessarily amateur friendly or desirable. So here are some ideas for anybody that has
contemplated trying to prepare something from terephthalic acid but was afraid to try:
1) Salts: the di-sodium terephthalate is easily prepared and from this can be prepared a wide range of generally sparingly soluble salts by double
decomposition. Some the rare earth salts are reported to have fluorescent and luminescent properties (1), (2).
2) Esterification: Terephthalic acid can be converted to its dimethyl or diethyl ester with the appropriate alcohol and either sulphuric acid or
gaseous HCl (5, 7). The resulting esters are crystalline solids. These esters are fairly easily half hydrolysed (5, 7, 9) to terephthalic acid
monomethyl/ethyl ester and it may be possible to generate the half amide by a modified process too, from which, via Hofmann’s degradation,
4-aminobenzoic acid ester may be available. The mono esters can also be converted to monoacid chlorides (7) and from these via treatment with ammonia
the monoamide. There is an SM thread by Picric-A on the this process but unfortunately it is lacking details of experimental procedures and he failed
to provide any in spite of repeated requests from other members to do so and his procedure from ammonium hydrogen terephthalate must remain
questionable, however, see the comments below. The 4-aminobenzoate ester is easily nitrated and the nitration products reduced with sulphide to
3,4-diamine and 3,4-diamino-5-nitrobenzoate esters which when treated with nitrous acid are converted to benzotriazole derivatives (my interest).
Other possible routes from the 3-nitro-4-amino ester include hypochlorite oxidation to a benzofuran derivative.
3) Nitration: Nitration is reported to require rather forcing condition with large excesses of fuming sulphuric and fuming nitric acid to yield the
mononitroterephthalic acid (only one isomer is possible) (3, 8). However, I think it would be worth trying this nitration with alkali nitrates and
conc. Sulphuric acid at moderately elevated temperatures instead. The reduction of the nitro compound to the amino compound and then via diazotization
to the hydroxyterephthalic acid has been reported (3); Wegschieder (8) also described the reduction of nitroterephthalic to the amino acid
(effectively an anthranilic acid derivative). The presence of the amino or hydroxy group may be sufficient to activate the nucleous to further
substitution and it is interesting to speculate on the possible de-carboxylation on further substitution in the manner of salicylic acid to picric
acid under trinitration to yield in this case 2,4,6-trinitro-3-hydroxybenzoic acid. It is possible that both carboxylic acid groups would be lost in
this process as the reference above (3) reports that bromination of 2-hydroxyterephthalic acid occurs readily forming 2,4,6-tribromophenol.
4) Mercuration: Terephthalic acid can be mercurated with mercuric oxide or mercuric acetate and then converted to the monohaloterephthalic acid by
treatment with the appropriate halogen (4). While the process sounds simple enough the very long refluxing (>10 days!!) and the production of much
unusable mercury bearing organic waste makes this process impractical as a preparative route.
5) Iso and terephthalic acids can be converted to their respective acid chlorides and then reduced to the corresponding dialdehyde (6). This route is
also of limited value to amateurs in view of the need for compounds such as thionyl chloride or phosphorus pentachloride.
6) Another possible routes to derivatives is the direct amidation by treatment of the free acid or ammonium salt with urea and sulphamic acid (10 but
the reference is not entirely clear as to whether the product is the mono or diamide, the former is implied but no supporting data is provided) or
with urea and boric acid (11) to the half or full amide. It may then be possible to convert the amides into the nitrile with ammonium sulphamate (12)
to give the 4-cyanobenzoic acid or 1,4-dicyanobenzene respectively.
The decarboxylation of terephthalic acid to benzene or benzoic acid has been discussed on this site in several threads but I have found no published
article specific to terephthalic acid for this process yet.
(1) Dalguebonne et al., Inorg. Chem., (2008), v47(is9) p3700. DOI; ic702325m (luminescent RE salts)
(2) Dalguebonne et al., Inorg. Chem. (2006), v45(is14) p5399. DOI; ic060241t (Erbium salts)
(3) Burkhardt GA. Berichte., (1877), v10, p145. (hydroxyterephthalic acid via nitration, reduction to amine and diazotization)
(4) Frank et al., JACS (1929), 51(9), pp2785- . DOI; ja01384a027 (mercuration)
(5) JOC (1960), v25(5), p817; DOI jo01075a039 (esterification and half hydrolysis)
(6) Rosemund and Zetsche, Berichte., (1921), v54, p2888. (reduction of iso and terephthalic acid to appropriate aldehyde via the acid chlorides)
(7) Cohen & Pennington, JCS, (1918), v113, p63. (diethyl ester, diacid chloride, monoethyl ester and monoester chloride of terephthalic acid and
nitrophthalic acid).
(8) Wegschieder R., Monatsch., (1900), v21, p621. (nitration of terephthalic acid and its salts)
(9) Wegschieder R., Monatsch., (1902), v23, p405. (nitroterephthalic acid esters and half esters)
(10) US 3663589 J Luecke, Process for the production of nitriles (-from carboxylic acids with sulphamic acid see example 4)
(11) SM thread “Benzoic acid, Carbamide>benzamide>aniline” http://www.sciencemadness.org/talk/viewthread.php?tid=4201
(12) Can J Research, J. L. Boivin, (1950), v28, p671, (Nitriles from amides & sulphamic acid)
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Ta-da!
Congrats Boffis! :-)
Your quite detailed work is warmly appreciated, thanks!
PET hydrolysis and terephtalic acid chemistry is something that bothers me a lot. It is so OTC.
Now you compiled a very good starting point to help me (and probably others) to pursue this route.
How do you cut up the bottles? With a sharp knife? Or do you have any special technique? How small the bits are? How long doeas it take to hydrolise
them?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Boffis, thanks for the excellent review. This forum needs more such topics.
I can add that terephthalic acid is the prototypical ligand for the synthesis of metal-organic framework materials (MOF), a class of coordination polymers.
Two randomly chosen (application oriented) review articles: DOI: 10.1021/cr9003924 and DOI: 10.1021/cr200190s
I'm often thinking why home chemists show practically no interest in coordination polymers. They appear so suitable for a few reasons. Some are very
easily synthesized (at least microcrystalline and solvated), there is a lot yet to be discovered (at least application-wise), their structure is often
very hard or impossible to determine, so that characterization by application is acceptable even for publishing... And if you go on to synthesize your
own multidentate ligands, then you are bound to learn a great deal of organic synthesis as a bonus.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Can 4-aminobenzoic acid (which probably could be made from terephtalic acid) self-condense to long chain linear polyamide ? If yes, supposedly the
polymer is conductive.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Pumukli, I found that the larger 1.5-2 litre soda bottles are best but I currently use the 600ml water bottles because we are handed a couple of
these every day on site. I remove the labels, which are just a plastic band, and then remove the neck and the thick crown at the base with a knife
because they are difficult to cut up and then chop up the remainder with a pair of scissors. I cut it into strips about 1cm wide and then into small
squares of about 1cm2, it takes ages to cut up 250g! I wondered if you could use a domestic blender once you had removed the top and bottom but I
didn't try it.
@Nicodem, its funny you mentioned the coordination polymers. I had never heard of them before I read the two papers on the rare earth compounds but
they are almost exactly what I was originally chasing. My original target was 2,5-dihydroxyterephthalic acid, since salicylic acid produces a nice
purple but extremely soluble complex with ferric iron I wondered if two salicylic acid groups on the same benzene ring might produce a similar but
less soluble complex by virtue of its desire to form 2 or 3D structures. However, my literature search seems to indicate that the
dihydroxyterephthalic acid is generally prepared either from 2,5-dibromo-p-xylene or via a double Kolbe reaction from hydroquinone (salicylic acid is
produced via the analagous reaction from phenol). I am actually quite surprised that an isolated carboxylic acid group can form sufficently stable
coordination compounds to build such 3D structures, I would have thought that the extra OH adjacent to the carboxylic acid group would have greatly
improved stability; maybe it does have I haven't tried it yet.
Incidentally; will researching terephthalic acid I stumbled across a paper that covers another topic that crops up regularly., that of preparing
phthalic acid by oxidizing hydrocarbons. I will have to look for the paper again but in essence it described the preparation of all three phthalic
acid isomer by oxidation of mixed isomer xylene and then separation of the three resulting acid by fraction solution and then crystallisation of the
barium salt. They enriched there feed stock by partial removal of the o-isomer with a 1.5m tall fractionating column (!) first but I think the process
is capable of manipulation to allow the use of "as bought" xylene to yield all three acids. I am looking into this at present.
@papaya, I'm sure its possible to polymerise it but it might be rather difficult. I don't know much about these things but maybe you could use the
techniques they use to create peptides from amino acids.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Thanks Boffis,
I tried Esterification of Terephthalic acid with Short-chain alcohols like Methanol , Ethanol and i have to say this is not possible by standard
method.
Finally i made esters of Terephthalic acid by acyl chloride route.
This is interesting for me to know:
P-Phenylenediamine from Terephthalamide is possible?
Does Hofmann rearrangement is possible for both amide in Terephthalamide at same time?
[Edited on 4-8-2015 by Waffles SS]
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
A New Process for the Production of p-Phenylenediamine Alternatively from Polyester Waste, Terephthalic Ester, or Terephthalic Acid
Hans G. Zengel , Manfred J. Bergfeld
Ind. Eng. Chem. Prod. Res. Dev., 1976, 15 (3), pp 186–189
DOI: 10.1021/i360059a009
Publication Date: September 1976
If someone could access (and post) it that would be nice! :-)
I can't.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@waffles; I am surprised you found it so difficult to esterify terephthalic acid by the Fischer process, it is described in several of the references
above so I would give it another go. I will certainly try it myself shortly.
By the way what method did you use to generate the acyl chloride? It looks like Pumukli's references answers your question about hofmann degradation
of terephthalamide.
@Pumukli, attached is you reference, very interesting indeed. It sounds as though only a small over pressure of ammonia is required so it may be
possible to rig up such equipment at home.
Attachment: Production of p-phenylenediamine from terephthalate esters Ind Eng Chem Prod Res Dev Zengel & Bergfeld 1976.pdf (449kB)
This file has been downloaded 1589 times
[Edited on 5-8-2015 by Boffis]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
@Boffis,
I made Terephthaloyl chloride by refluxing excess Thionyl chloride with terephthalic acid and then distilling the product.





I also sent requested @Pumukli reference by private message to him according to copy right law 
[Edited on 5-8-2015 by Waffles SS]
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I'm glad that I may helped you with the reference. Thanks, I got it, read it.
Regarding the first stage, the ammonolysis, it works as I expected (silently). My original idea was that PET is an ester and esters usually give amids
when reacted with NH3. I hoped that terephtalic acid is no exception and glycolic esters are also working in this regard.
Now the overpressure issue.
I think it may be circumvented by using much finer starting material. Instead of PET chips I plan making PET "grindings" with some sort of low speed
grinder machine. It may also take ages to produce a sizable ammount this way but the much increased surface area may outweigh the sweat and tears.
Ball milling with dry ice would probably be even better, but is not realistic for me.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Quote: Originally posted by Pumukli  |
Regarding the first stage, the ammonolysis, it works as I expected (silently). My original idea was that PET is an ester and esters usually give amids
when reacted with NH3. I hoped that terephtalic acid is no exception and glycolic esters are also working in this regard.
|
Once when storing 15% ammonia in a PET bottle it "corroded" away just in a week, nearly up to making holes in it and all covered in white specs
,especially ABOVE the level of liquid ! As ammonia is not as "basic" as say NaOH, can I speculate that what formed was amide(s)?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by papaya  |
Once when storing 15% ammonia in a PET bottle it "corroded" away just in a week, nearly up to making holes in it and all covered in white specs
,especially ABOVE the level of liquid ! As ammonia is not as "basic" as say NaOH, can I speculate that what formed was amide(s)?
|
Yes,
Ammonolysis of esters lead to Amides
Aminolysis of PET by Methylamine and Ethylenediamine at room temperature going faster than Ammonia and even faster if you use small amount of Phase
Transfer Catalyst like TBAB or CTAB(google it to find interesting articles)
[Edited on 6-8-2015 by Waffles SS]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Waffles, Nice to have access to thionyl chloride. I might try with PCl5 to give terephthalylchloride and phosphorus oxychloride. But with the number
of published descriptions of esterification of terephthalic acid with methanol or ethanol and sulphuric acid it must be possible. I am going to try it
with anhydrous methanol and possibly azeotropic water removal after a reasonable reflux period.
Reading the Zengel and Bregfeld paper again, they refer to the glycol not only as a solvent also as a catalyst. This and Papaya's comments make me
wonder if you could just "oil" the PET chips with glycol and pack them in a glass vessel and pass ammonia gas through it slowly over a period of a
week or two at atmospheric pressure. Or possibly using a Schott type bottle in an oil bath at 140 C for just a day or so.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by Boffis  | @Waffles, Nice to have access to thionyl chloride. I might try with PCl5 to give terephthalylchloride and phosphorus oxychloride. But with the number
of published descriptions of esterification of terephthalic acid with methanol or ethanol and sulphuric acid it must be possible. I am going to try it
with anhydrous methanol and possibly azeotropic water removal after a reasonable reflux period.
|
You can use S2Cl2 instead of Thionyl chloride(also i have access to Riedel-de Haën S2Cl2  ) )
Esterification of Terephthalic acid with methanol or ethanol is possible if you can bring out formed water from reaction
This is difficult because Methanol and Ethanol have lower bp than water(my suggestion is using Molecular sieve 3A or using large amount of Sulfuric
Acid as water absorber)
[Edited on 6-8-2015 by Waffles SS]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Waffles, one way to get the water out is to use a large excess of dry ethanol. After refluxing for some time the excess alcohol is distilled off, it
will remove the water as the azeotrope ethanol-water mixture. I don't think this works with methanol.
It may also be possible to remove the water by co-distillation with an immisible solvent such as hexane.
The paper cited above suggest that after an adiquate reflux period conversion reaches at least 70% ester and since the unreacted acid is recoverable I
don't see am big problem.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Use Chloroform for making ternary azeotrope(Water + Methanol + Chloroform=b.p.azeo. 52˚C)
https://en.wikipedia.org/wiki/Azeotrope_tables
I usually use below apparatus for making this type of ester and another Methyl or Ethyl ester(Oxalate,Tartarate ,..)
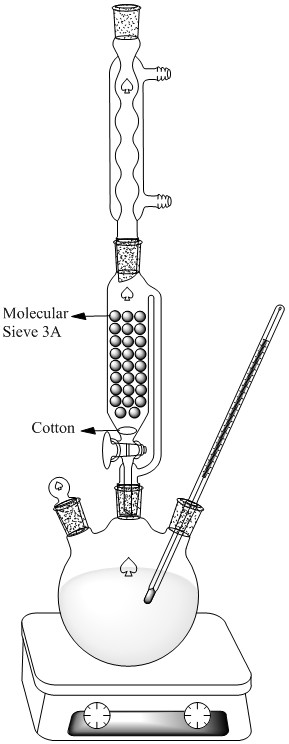
At end you should wash your ester with Sodium Carbonate solution for removing unreacted acid and mono ester of Terephthalic acid
[Edited on 7-8-2015 by Waffles SS]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Waffles; interesting idea. I was planning to try n-hexane as a substitute for benzene. My only worry about chloroform is how reactive is it towards
the sulphuric acid-alcohol mixture?
I have the equipment to use the zeolite assisted esterification but how much zeolite do you need per gram of water that will be formed?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Use PTSA(Para-toluenesulfonic acid) instead of sulfuric acid as catalyst
I dont have experience on zeolite but i prefer Molecular sieve
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Waffles; most molecular sieves ARE zeolites!
I thought I would have a go at nitrating terephthalic acid today, here is what I did;-
I added 4g of dry terephthalic acid to 20ml of 96% sulphuric acid and then added 2.5g of finely ground dry sodium nitrate. After mixing I let it stand
for 30 minutes but nothing seemed to happen so I put it on a hotplate that I had used earlier but which was still warm. After another 30 minutes to
temperature was such that the beaker was uncomfortably hot to the touch but still nothing appeared to happen so I turn the hotplate on again and
heated it further until the the mixture began to to simmer and give off small amounts of nitric acid smelling vapors but still not visible reaction!
At this point I gave up and left the mixture to cool down. When almost cold I scraped the viscous white mass into 100ml of cold water. The result was
an immediate bright yellow colour and much insoluble material, the whole resembled a suspension of sulphur powder!
I heated the mixture almost to boiling to try and dissolve the precipitate but to no avail, so I cooled it again and then filtered it. The filtrate is
a beautiful bright yellow colour but the solid is white and looks like the original terephthalic acid. I will investigate the solid further but I
could not recover anything from the yellow acid residue.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Sorry you are right
I didnt know my 3A molecular sieve made of zeolite
[Edited on 20-8-2015 by Waffles SS]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I was so intriged by Papaya's comments about the "corrosion" of a PET bottle by aqueous ammonia I just has to try it. The results were slow but
amazing; no pressure vessel, no gaseous ammonia or high temperatures just patience!
I took the thicker parts of a PET water bottle from the top and bottom to see how far the process would penetrate. I smashed them with a hammer to
make them fit through the top of a glass soda drink bottle but would wedge in the top out of contact with the liquid, I would estimete the weight to
be 3 to 4g. I then added 50ml of 32% ammonia solution so that the plastic was wetted but most of the liquid drained to the bottom of the bottle and
replaced the lid. I then left the bottle on a shelf in my office for 3 weeks while I was on holiday. When I returned the plastic had turned white and
partly crumbled and all was lying in the ammonia liquid at the bottom of the bottle. On shaking much of the white plastic was reduced to white crumbs
which suggests that in the thinner sections were completely altered though the thick disc at the base and the lip on the neck are not completely
altered.
 White altered PET after 3 weeks White altered PET after 3 weeks
I will let it stand for a further 3 or 4 weks and then filter off the solids, wash them and then gently grind them to see how much PET is left.
I think that if 15% ammonia solution works then you probably only need a 30-40% excess af ammonia solution at 32%. This coupled with the use of only
the thinner wall sections of the PET bottles could be an interesting "home" route to various compounds. You just need to be patient.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I recall hearing something about PET bottles that save costs now by using double layers, one of a less expensive material.
Here's some article about special purpose PET bottles that use multi-layers, just on the off chance the ammonia treatment produces different results
at times with various PET samples.
http://www.ptonline.com/articles/barrier-pet-bottles
Another
http://www.gasbarriertechnologies.com/petbottles.html
[Edited on 4-9-2015 by Morgan]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Good to hear about your success Boffis, what I also remember from my accident is that I didn't want to throw away that ammonia which stayed in PET
bottle considering it must be nearly pure, so I transferred solution into the glass bottle. After a day a thin layer of well formed needle-like
crystalls precipitated from totally clear ammonia solution! Seems that, whatever product it gives, it is only marginally soluble in water, and it may
be that what you obtained after weeks of reaction in form of white crumbled stuff is not the original plastic anymore, but the product!
One more idea at the end: pet bottles melt when you heat them. When solidified it's no more elastic, but can be crumbled/milled easily into powder,
which must reduce reaction time down to several hours/days.
[Edited on 4-9-2015 by papaya]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you Boffis for this excellent work!
Here are my 50 cents... since I also have thought for years about the possible uses of PET and terephtalic acid. 
1°) If one has access to NaN3 or to N2H4 he could perform:
°Cutrius reaction via Ar-CO-NH-NH2 and HNO2
°Schmidt reaction with NaN3 and H2SO4
Partial reactions would lead to HO2C-Ar-NH2 and complete reaction would lead to H2N-Ar-NH2
From there diazotation is possible with a lot of possible products.
2°) H2N-Ar-NH2 could be reacted with Cl-CO-Ar-CO-Cl (terephtalyl chloride) or with MeO-CO-Ar-CO-OMe (dimethyl terephtalate) to yield kevlar
(-NH-Ar-NH-CO-Ar-CO-)n
3°) H2N-Ar-NH2 reacted with aqua regia (HNO3/HCl) will yield chloranile (O=C(-CCl=CCl-)2C=O), with HBr/HNO3 bromanile and probably with I2/HNO3
iodanile.
The halogens are very labile and may lead to halogen exchange reaction; for example tetrabromo-p-quinone (bromanile) will exchange 2 I
(diiodo-dibromo-p-quinone) upon reaction of KI/EtOH and finally 4 I (iodanile) if longer exposure time, heat, reflux and more reactants.
The halogen exchange of chloranile, bromanile and iodanile is characterized by the easy replacement of each halide by azide group upon contact with
NaN3 water solution forming the extremely dangerous tetraazido-p-quinone (intermediary stages are possible with mono, di, tri azides). This was
covered by Axt in this forum.
Same is seen with NaNO2 what forms nitranilic acid (2,5-dihydroxy-3,6-dinitro-p-quinone) forming primary explosive salts like silver and lead
nitranilates (the substitution passes maybe via a transcient tetranitro-p-quinone that hydrolyses spontaneously to the dihydroxy-dinitro compound).
Maybe other easy substitutions are possible like OH(-), C#N(-), NH3, NH2OH and N2H4 (this last one will probably reduce the quinone)...to test also
amines like CH3-NH2, (NH2)2C=NH, ...
4°) Reaction of aqua regia or of HBr/HNO3 on terephtalic acid is not clear...
-maybe it remains unaffected
-or it is partially halogenated (and maybe decarboxylated) --> halo (dihalo, trihalo or tetrahalo) terephtalic acid and maybe polyhalobenzenes and
polyhalobenzoic acid
-or decomposed into chloropicrin (Cl3C-NO2) or bromonitromethanes (CH2Br-NO2, CHBr2-NO2, CBr3-NO2).
The halopicrines are good ways to guanidine via reaction with concentrated ammonia solutions
Cl3C-NO2 -NH3 conc-> (H2N)2C=NH.HCl + NH4Cl + N2 + H2O
Chloropicrine may be dehalogenated and reduced by H2S in basic media to dichloronitromethane, chloronitromethane, nitromethane, methylamine and
finally formaldehyde-methanol.
5°) Reaction of iodination (periodination) reagents (HNO3/I2, Cl-I, I2/SO3) allows one to get acces to iodo-, diiodo-, triiodo- and
tetraiodo-terephtalic acid (this last one is a X-Ray contrast media for blood system or urinary system medical study).
Under harsh conditions decarboxylation may occure to yield pentaiodobenzoic acid and hexaiodobenzene...
6°) Hunsdieker reaction is not working well with terephtalic acid (less than 2% yield) but well with benzoic acid what will yield chlorobenzene or
bromobenzene from silver benzoate (see point 7°) and Cl2 or Br2 in refluxing CCl4 or other suitable solvents.
7°) Thermolysis (425°C) of dry silver terephtalates generates a lot of products like benzoic acid, benzene, biphenyl as major products and
terphenyl, quaterphenyl, quinquephenyl, 3,4-benzocoumarin as minor products.
See in requested documents "Thermal and Photochemical Decomposition of Silver Carboxylates", requested by me.
So from this tread, it is obvious many things (if not everything) can be done from easily got OTC PET...
The only limits are:
-your mind 
-time 
and as usual 
-money 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Papaya, yes I'm pretty sure you're right about the white product being the diamide and it also appears that your observation that moist ammonia gas
is more aggressive than the solution is correct. With this in mind I am going to try some further larger scale experiments where the PET is isolated
from the ammonia solution.
@Philou, thank you for the ideas you posted. There are some interesting possibilities here. I wonder if hydrazine solution would be more aggressive
than ammonia solution? I have some 60% hydrazine hydrate solution (about 40% hydrazine), the aim would be to produce the dihydrazide directly from
PET. The lower vapor pressure of hydrazine over this solution means you can probably reflux it at atmospheric pressure with minimal loss of hydrazine.
The direct halogenation would also be interesting if it can be made to work but given the inertness of terephthalic acid to nitration I think very
harsh conditions will be required though once introduced these halogen are fairly reactive and can be replaced by OH using KOH.
Update on the ammonia experiment: After a vigorous shaking most of the white alteration product became detached from the residual PET but the the
thicker parts of the threads and neck still remain see photo.

The PET in ammonia after about 4.5 weeks, fragments of threads and the star from the base are clearly visible.
|
|
|
| Pages:
1
2
3
..
5 |