| Pages:
1
2 |
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Glassblowing
I've seen a little bit of interesting discussion on glassblowing to make your own RBFs and other things, and was wondering what sort of things are
needed to get started?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Do a youtube search on Lab Glass Blowing. There are dozens of very cool videos. There is quite a lot of it you can do at home or in a glass blowers
co-op. This vid shows a basic set up, and demonstration.
https://www.youtube.com/watch?v=CGngoVau4ME
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
The main challenge is the gas and oxygen to run a real glass torch, plus the torch, those all add up to a good bit, unfortunately, otherwise I would
have one. I sent some stuff to the guy said that he would repair stuff, but I have not gotten anything back yet.
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Could a propane/MAPP gas torch work for very small-scale glasswork?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Very small-scale. It takes a lot of practice to make sure that the heat of the flame is focused on just the right part of the glass, but even then
you won't be able to do anything very large. Sealing the necks of ampoules is the only "glassblowing" I do frequently and a propane torch is hot
enough if it's focused on the right spot, but anything much larger than that or similarly sized glass tubing will need a real oxygen-fuel torch. I
find MAPP gas, or at least the torch I have, is not preferable for any sort of glassblowing because the flame is much less focused in one spot whereas
a propane torch has a very sharp, well defined flame.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by gdflp  | | I find MAPP gas, or at least the torch I have, is not preferable for any sort of glassblowing because the flame is much less focused in one spot
whereas a propane torch has a very sharp, well defined flame. |
That IS a result of your torch. and not the gas.
Mapp gas is simply hotter than propane, and in reality much easier to use for small scale glass work. The work is faster, and you use less gas, saving
money in the long run. 
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I agree. A Bernz-O-matic torch will give a fine point with MAPP.
--------------------------------------------------------------------------
I looked hard at Bernz-0-matic's oxy-propane torch sold by Home Depot for ~$60. But the customer comments were only about 50% favorable. Comments
ranged from "...never should have been put on the market..." to "...did the job, what you would expect...." The major problem centered around the
fine control of the oxygen. It does not use the typical regulator but instead has a multi-turn needle valve.
The Bernz torch w/MAPP will melt borosilicate, barely. It would probably do a good job with lime-soda glass. But who uses that anymore - maybe for
some test tubes.
[Edited on 20-4-2015 by Magpie]
[Edited on 20-4-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Kitsune1
Harmless

Posts: 19
Registered: 10-3-2015
Member Is Offline
Mood: No Mood
|
|
I have managed to do a few things with a simple alcohol lamp, a few days ago I converted a shattered borosilicate burette into an air cooled condenser
column (it works great, used it today with MeOH) all without using my torch, It just takes a very long time with heating, you need to be careful and
sometimes your joints might pick up small amounts of carbon. I often just use an alcohol lamp to make small pieces of apparatus from tubes, pipettes,
ampoules and even the small glass ampoules that you can find inside a long thin glowstick (it actually works really well for making Tetraphosphorus as
long as you are careful when you make the apparatus and also for ampouling very small quantities of compounds in either solid, liquid or gaseous
states).
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Kitsune1  | | I have managed to do a few things with a simple alcohol lamp, a few days ago I converted a shattered borosilicate burette into an air cooled condenser
column (it works great, used it today with MeOH) all without using my torch, It just takes a very long time with heating, you need to be careful and
sometimes your joints might pick up small amounts of carbon. I often just use an alcohol lamp to make small pieces of apparatus from tubes, pipettes,
ampoules and even the small glass ampoules that you can find inside a long thin glowstick (it actually works really well for making Tetraphosphorus as
long as you are careful when you make the apparatus and also for ampouling very small quantities of compounds in either solid, liquid or gaseous
states). |
Sounds like fun, actually!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Using a Bernz0matic torch on MAPP I have made some very rugged looking annular sparge tubes. The fusing is solid with no visible seam. But these
eventually fail in testing as the inner 6mm tube snaps off right at the base of the weld. I suspect that the failures may be due to inadequate
annealing, a subject about which I am just learning.
Any suggestions or guidance on annealing would be appreciated.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jylliana
Hazard to Others
  
Posts: 126
Registered: 3-10-2014
Location: The Netherlands
Member Is Offline
Mood: Bubbly ^-^
|
|
I haven't read the full thing, but it may be interesting for people here to read
A Handbook of Laboratory Glass-Blowing by Bernard D. Bolas
Download free and legal, afaik.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I have done glass blowing in the past. but it was art and pipes for a glass blower that serviced shops in the area. he barely paid me, but I did
learn a lot. what you guys are describing with the map gas torch and all,... no where near as easy as a real torch set up.... which wasn't easy at
all.
for one, there was really great control, and 2 separate torches built into one device. it had a head roughly 1.5" square for heating a large area,
and a small head directly above it that was the fine flame. you could run both at the same time and adjust individually to heat and work larger areas
or thicker glass. while adjusting O2 and propane for oxidizing/reducing flame or running a lot of O2 for a cool flame for delicate work. the oxygen
came out cold if you turned it up on high. up to 3" borosilicate tube on a regular basis. we used a huge standalone oxygen take about 5' tall and 2'
across with propane tanks you might find on an RV or large BBQ. ( we also lived/worked in a prop 215 friendly county ) I didn't jump on the medical
band wagon but we( mendocino county) were some of the first to use medical legally, and it was a legitimate business. so please don't bust my chops
about that. all above board.
we ALWAYS wore the rosy color eye protection to block out IR, and shield you from shattering glass. we worked on a standard oak desk with two layers
of concrete board atop it. on this was an assortment of graphite tipped tools. there was a large graphite plate to set stuff down on while using
your hands for other things. we had the blow tube routed around the flames in PCV with rubber hose to the mouth and back of the work piece with a
cork. for blowing out a sphere after gathering some tube into a larger lump at one end. there were tweezers for pulling off extra borosillicate when
you gathered too much, ya we used the good stuff. metal shears for trimming, cutting and making petals in hot glass tubing ends for decorative
pieces. there were 2 graphite blocks. one with half spheres of different sizes cut into it, and one with horisontal half cylinders for necking down
or trueing. all the extra's and still it was no easy task to make repeatably good work. besides the clear glass we used colored stock for the
designs and dichroic glass for glitter like effects. "millies" were rods of glass with a design already built in. like a peace sign, skull, flower
or smiley face. the rod was the same down the entire lenght if cut into thin wafers. you would make a hot spot on the glass and stick it on there,
after cooling a bit, you would snap off the rod leaving a thin layer of the design stuck to the piece. we had an electric kiln to anneal the pieces.
which still shattered occasionally, for no reason = not easy.
I got to fume the pieces with gold and silver, made some colored 3" butterfly decorations on the end of a rod to be placed in a flower pot, several
small jars with colored designs, many many crappy pipes that were just fumed, quite a few that were full colored, some that were fumed and colored...
but he stopped paying the employee that was teaching me and had been working for him for a few years... so I quit. she did nothing wrong. he was
just into his hobbies way too much to support employees... all in all it was really fun and I wish I had a set up to play with again. surely
wouldn't be making art or pipes any more.
the blower was talented, made a bubbler that was almost 3 foot long with a dragons head receptacle for the "tobacco". and all kinds of designs, warts,
and a scale pattern scheme. in the middle there was a clear section about 10" long that he put a dragons head with long waving tongue INSIDE. when
you drew through the thing, the smoke would percolate throught the body and come out the dragons mouth *visibly* swirling through the clear part as if
the dragon was blowing fire. it then went to the mouth piece and out. he did the inside out version of things where the design was done on an opened
up tube( flared like a funnel) and just about any other trick you see in a high times magazine. he promised but never came through with some beakers
etc. before I knew where to find any. this was about 10 years ago.
simple glassware on a makeshift torch, not out of the question, but repeatability, quality and durability,... I doubt that. the annealing was really
important. one of the pipes was dropped off the front of a bluff overlooking the ocean. sloping quite steeply, 75' if it was an inch, banging off
rocks and all, barely a chip... now that is bullet proof. think he is living in the bay areas here in cali now for some time, 7 yrs, still blowing
glass. if it weren't for the start up costs, I would love to do that again. the torch alone was 450$ back then though. who knows you may find one
for cheap some where used.
[Edited on 24-4-2015 by violet sin]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
polariscope
I have assembled a new sparge tube using Simax borosilicate as in previous tries. Again I used MAPP gas and a Bernz-0-Matic torch. Not being an
oxy-torch the heat provided is marginal. But the weld looks good and I carefully annealed the assembly. At this time I am assembling a polariscope
so that I can check for residual stress. If this device proves out I will post the results.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah... annealing. That's something I definitely need to work on. The simple work that I've done with shaping small tubes in a Bunsen flame didn't
really require much work as far as annealing goes, but when I tried attaching a piece of tubing to a hole I drilled into a broken test tube (all for
practice) using a MAPP torch, I was able to fuse the pieces well, but it was far too hot, and shattered after cooling. Would quickly switching from
MAPP to propane for a while before slowly removing it from the heat be sufficient to allow it to cool slowly enough, or would I need to use an oven?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I think you could help the glass cool slowly by just wafting the torch over the worked area for a few minutes with a decreasing frequency.
Possibly a better method would be to place the work under a fiberglass blanket, or better yet submerge the work under 2-3" of vermiculite until cool.
This last method is something I read on a glass blowing site.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Erm, hasn't glass technology been around for a while ?
Heat and Brains are required.
The early glass-blowers had no access to gas torches yet managed incredible structures ( e.g. a tube ).
Gas torches make it whole lot easier, granted, yet you can still do this at home.
Here's a Challenge : make something useful (for chemistry) from a bottle/bottles you found in the garbage.
Long Steel pipe, Barbecue, Charcoal, Hairdryer : give it a go !
(hint: use a long steel tube (fence post) so your hairdryer doesn't melt)
One suggestion would be a gas-wash bottle.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by Kitsune1  | | I have managed to do a few things with a simple alcohol lamp, a few days ago I converted a shattered borosilicate burette into an air cooled condenser
column (it works great, used it today with MeOH) all without using my torch |
That's unlikely.
Borosiliate glass softening point is in the 800 C range. I had a lot of difficulty in melting shut a test tube with a propane torch. Borosilicate
glass requires a oxy-gas torch to reach large volumes of heat.
Of course, you can melt fine copper wire in a methanol flame as I often did when I was a teen.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I finished making a polariscope today. This is to be used for examination of glass work for residual stress. I followed the instructions found at
the link below except that (1) I used 86mm linear polarizing filters vs his 72mm filters, and (2) I used 48 LEDs vs his 44 LEDs for the light source.
http://www.rhunt.f9.co.uk/Glass_Blowing/Polariscope/Polarisc...
The picture below shows my completed polariscope:

completed polariscope
The next picture was taken with the two polarizing lenses aligned to maximum light. The LEDs are quite visible through the Lucite (60% transmittance)
diffuser. The cone of of light is only 12° for these LEDs which makes them quite distinct.

polarizing lenses aligned
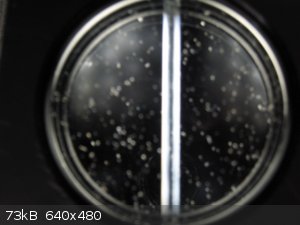
The next picture shows the polariscope with the polarizing lenses crossed for maximum darkness. The photo is poor. In my view it is totally dark.
The flecks of dust are bits of construction debris (cuttings) that I haven't completely removed yet.
Attachment: phpyCobug (92kB)
This file has been downloaded 612 times
polarizing lenses at 90°
The last picture shows an annealed sparge tube. I could see no stress pattern. You can clearly see the extended inner tube and where the tubes are
fused. The orange color on the end is where I had placed a short piece of 8AWG copper wire to prevent closure of the inner tube. After the fusing
the wire was removed by dissolution with nitric acid.

annealed sparge tube
I also looked at a sparge tube that had not been annealed. It showed considerable stress lines. I will try to post some better pictures later.
[Edited on 16-5-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Magpie. I am continually impressed by the gidgets and gadgets you construct. Nice work.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you j_. I only build gadgets that are needed to support my chemistry hobby, or solve some other problem. I don't build them as a hobby per
se. But they are usually fun and always educational. Sometimes the structural aspects can be tedious and expensive. The polariscope is a good
example. Try to find (locally) spring clips to support the glass plate that supports the upper polaroid lense, for example. Cost on the internet:
pennies, shipping cost: $8. I ended up making the 4 spring clips from two bicycle handle bar clips by cutting them in half and drilling mounting
holes. Spring steel is extremely hard.
Dirt cheap polaroid lenses from China made the polariscope feasible.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's some more pictures taken of the polariscope in use. I tried to clean all the surfaces with glass cleaner, wiping with bilbous paper which is
used to clean glass slides. Obviously it didn't do any good.
The first picture is the end of a stirring rod showing the stress in the end. I'm presuming this is residual stress resulting from the manufacturing
process. This is a used rod. I should have checked a new rod.

stirring rod stress
The next picture is that of an unstressed 5 mm borosilicate glass tube.

unstressed glass tube
The last picture is of that same tube experiencing bending moment stress (using my hands).
bending stress in tube
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
SimpleChemist-238
Hazard to Others
  
Posts: 147
Registered: 28-9-2014
Member Is Offline
Mood: Chlorine Trifloride Flame Thrower
|
|
I was making spirals with a propane torch today, lots of fun.
We are chemists , we bring light to the darkness. Knowledge to ignorant, excitement to the depressed and unknowing. we bring crops to broken fields
and water to the desert. Where there is fear we bring curiosity.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
I want to say that I sent some items to theglassguy, and after a little while, I got most back, most repaired, with one or two items were not fixable,
one got fixed them broke, and one got broke in repairing it, and he remade it. The repairs look reasonable, some I cannot really tell that they were
even repaired.
So I just wanted to post that. I would try to get a real email address and keep in closer contact, it took a while and I was not sure what was
happening until done. But since it was all broken then, it was not much of a risk to try out. Not sure what his schedule is like, but I just
wanted to give him cudos for helping me.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I presume this is your reference DrBob http://www.sciencemadness.org/talk/viewthread.php?tid=37974
I think I might have taken advantage of that offer if I had been in a different location.
Nice to get some results back. I wondered how that had all worked out.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Yes, thanks for posting, I was in a hurry and did not have to look up the old thread, but that is it. I just wanted to acknowledge his good work.
Its been a bit crazy since my travel, still trying to get my act together, and answer a few emails and u2us.
Bob
|
|
|
| Pages:
1
2 |