| Pages:
1
2 |
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Doubts about H2O2 reduction potential
Looking at standard reduction potentials, the following reaction has a standard reduction potential of 1,78 V
H2O2 + 2H+ +2e- <=> 2H2O
which would make H2O2 a formidable oxidant. Yet, whenever I have an acidic solution of H2O2, most of the
time what happens is that the H2O2 gets oxidised to H2O and O2, even when reacting with supposedly
"weaker" oxidants.
When I put two oxidants together, what I expect is that they either don't react or that the stronger oxidant oxidizes the weaker one, IF they can
further be oxidized. In the case of H2O2, any mild oxidant will do the job.
Why? According to that table, H2O2 should be a monster of oxidant and yet it is used as a reductant/oxidant depending on the
situation. Why such misleading behaviour?
Also, I used H2O2 back then to oxidize Pb2+ to PbO2 and it actually worked. I had a solution of
Pb(NO3)2 in diluite HNO3 to which I added a solution of H2O2 and I actually saw the formation
of a red solid (PbO2) which was accompained by the bubbling of what supposedly was oxygen. But this made little sense to me. If indeed the
H2O2 had oxidized Pb(II) to Pb(IV), then H2O2 should have been reduced to water and not oxygen. This is
what should have happened:
H2O2 + 2H+ +2e- <=> 2H2O
Pb2+ +2H2O <=> PbO2 +4H+ +2e-
----------------------------------
H2O2 + Pb2+ ---> PbO2 + 2H+
but instead the reaction was accompained by the bubbling of oxygen, as if the freshly formed PbO2 was oxidizing the
H2O2 to O2, what the fuck?
Moreover, the only time I saw H2O2 acting as a "true" oxidant was when it was in basic solutions and not acid ones.
Can you shed some light on the ignorant one? Thanks
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Perhaps what happened with the lead oxidation is that some of the hydrogen peroxide was decomposed by the lead dioxide. This could give the appearance
of the reaction forming oxygen, even though it really didn't.
Although I don't know what's happening with regards to H2O2 behaving in as either an oxidant or a reductant depending on the situation I can tell you
that H2O2 oxidises Fe(II) to Fe(III) in acidic solutions, so it definitely can act as an oxidant under acidic conditions.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am with you on that one Metallus.
Just a couple of hours ago I was demonstrating the production of Cl2 and Br2 from NaCl and NaBr. I was using excess peroxide acidified with sulfuric
acid in both cases. I checked the reduction potential table just before hand and reasoned that H2O2 should be more than adequate for the job. Great
result on the Br2 but nothing much to speak of with the Cl2. I ended up throwing a spatula of KClO3 into the mix to get things going. Even though
ClO3- --> Cl2 has a reduction potential of 1.49 compared with peroxide's 1.78. Hardly a fair test since the chlorate produces its own chlorine
gas as well. I could have used permanganate (1.51V) except that I didn't want any colouration.
So I echo your question -- why is the actual performance of H2O2 as an oxidant so much less than what would be expected looking at the published
values?
(If I was to hazard a guess at the answer I would go for prohibitive kinetics and that the addition of a catalyst would bring the peroxide into line.)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I hope the following will clarify it, by means of an example.
In alkaline conditions, H2O2 oxidises Cr(III) to chromate (CrO<sub>4</sub><sup>2-</sup>, Cr(VI)) easily.
And yet that same Cr(VI), in acid conditions (it is then dichromate, Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup> H2O2 reduces Cr(VI) back to Cr(III). H2O2 reduces Cr(VI) back to Cr(III).
What H2O2 does simply depends on pH.
H2O2 + 2 H+ + 2 e === > 2 H2O
2 H2O === > 2 H+ + 2 OH-
---------------------------------------- (sum)
H2O2 + 2 e === > 2 OH-, in alkaline conditions, acts as an oxidiser.
H2O2 === > O2 + 2 H+ + 2 e, in acid conditions, acts as a reducing agent.
It behaves also like that with Ce(III) to Ce(IV) which happens with H2O2 in alkaline conditions. But Ce(IV) is reduced back to Ce(III) by H2O2 in acid
conditions.
[Edited on 23-2-2015 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by blogfast25  | I hope the following will clarify it, by means of an example.
In alkaline conditions, H2O2 oxidises Cr(III) to chromate (CrO<sub>4</sub><sup>2-</sup>, Cr(VI)) easily.
And yet that same Cr(VI), in acid conditions (it is then dichromate, Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup> H2O2 reduces Cr(VI) back to Cr(III). H2O2 reduces Cr(VI) back to Cr(III).
What H2O2 does simply depends on pH.
H2O2 + 2 H+ + 2 e === > 2 H2O
2 H2O === > 2 H+ + 2 OH-
---------------------------------------- (sum)
H2O2 + 2 e === > 2 OH-, in alkaline conditions, acts as an oxidiser.
H2O2 === > O2 + 2 H+ + 2 e, in acid conditions, acts as a reducing agent.
It behaves also like that with Ce(III) to Ce(IV) which happens with H2O2 in alkaline conditions. But Ce(IV) is reduced back to Ce(III) by H2O2 in acid
conditions.
[Edited on 23-2-2015 by blogfast25] |
Are you sure about this? The reaction
"H2O2 + 2 e === > 2 OH-, in alkaline conditions, acts as an oxidiser."
produces hydroxide, so I would imagine that this is favoured under acidic conditions where the concentration of hydroxide is low.
That same reaction can also be equivalently written as:
H2O2 + 2 e => 2 OH-
2H+ + 2OH- <=> 2H2O (water dissociation)
------------------------------------------(sum)
H2O2 + 2e- + 2H+ => 2H2O
Which makes it clear that this is favoured under acidic conditions.
Similarly, "H2O2 === > O2 + 2 H+ + 2 e, in acid conditions, acts as a reducing agent." I believe is favoured under alkaline conditions.
This is why hydrogen peroxide decomposes rapidly into oxygen and water under alkaline conditions and is reasonably stable under acidic conditions
(free of catalysts).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
deltaH:
Both assertions are based on direct, multiple observations with Cr(III)/Cr(VI) and Ce(III)/Ce(IV) oxidations/reductions with hydrogen peroxide. Verify
them by all means.
The preparation of chromate with peroxide has that drawback that if there's any peroxide left after the oxidation, it's part reversed when you
acidify!
How alkaline is alkaline and how acidic is acidic? How long is a piece of string?
Having said all that, H2O2 works also as an oxidiser in acid conditions, see e.g. Fe(II) to Fe(III). It's the overall cell potential that must be >
0 for anything to work.
[Edited on 23-2-2015 by blogfast25]
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | I hope the following will clarify it, by means of an example.
In alkaline conditions, H2O2 oxidises Cr(III) to chromate (CrO<sub>4</sub><sup>2-</sup>, Cr(VI)) easily.
And yet that same Cr(VI), in acid conditions (it is then dichromate, Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup> H2O2 reduces Cr(VI) back to Cr(III). H2O2 reduces Cr(VI) back to Cr(III).
What H2O2 does simply depends on pH.
H2O2 + 2 H+ + 2 e === > 2 H2O
2 H2O === > 2 H+ + 2 OH-
---------------------------------------- (sum)
H2O2 + 2 e === > 2 OH-, in alkaline conditions, acts as an oxidiser.
H2O2 === > O2 + 2 H+ + 2 e, in acid conditions, acts as a reducing agent.
It behaves also like that with Ce(III) to Ce(IV) which happens with H2O2 in alkaline conditions. But Ce(IV) is reduced back to Ce(III) by H2O2 in acid
conditions.
[Edited on 23-2-2015 by blogfast25] |
Yes, that is indeed what I observed sperimentally:
| Quote: |
Moreover, the only time I saw H2O2 acting as a "true" oxidant was when it was in basic solutions and not acid ones.
|
But then, how do you explain the fact that the reduction potential table lists H2O2 in acidic conditions as one of the best oxidants while the basic
version is lower on the table and supposedly not even strong enough to oxidize Cr III to Cr VI? It's like it was reversed!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Metallus  |
But then, how do you explain the fact that the reduction potential table lists H2O2 in acidic conditions as one of the best oxidants while the basic
version is lower on the table and supposedly not even strong enough to oxidize Cr III to Cr VI? It's like it was reversed!
|
For starters, see if you can find the half potentials for the reduction (as an oxidiser it gets reduced!) for H2O2 in acid and alkaline conditions and
compare.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | | For starters, see if you can find the half potentials for the reduction (as an oxidiser it gets reduced!) for H2O2 in acid and alkaline conditions and
compare. |
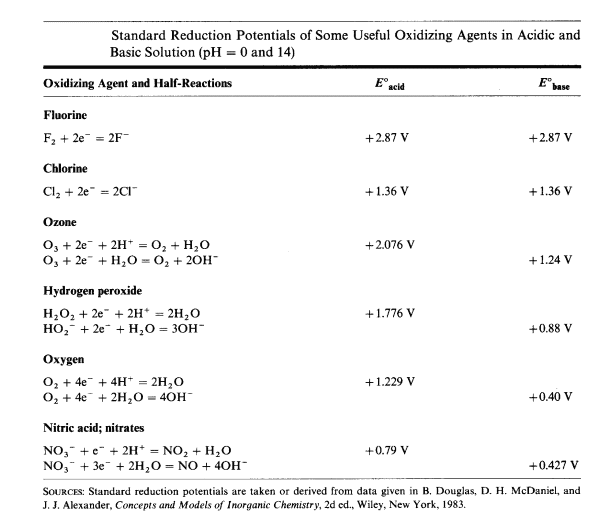
[Edited on 24-2-2015 by Metallus]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Metallus:
Very nice, ta.
The case of Cr(III) may be a bit of an anomaly. I know that despite listed values for reduction potentials, aqueous Cr(III) is very difficult to
REDUCE to Cr(0). This may be due to complexation (Cr(III) is a prolific complex former). Perhaps this explains also why Cr(III) is difficult to
OXIDISE in ACID conditions? In alkaline conditions it's mainly Cr(OH)<sub>4</sub><sup>-</sup>, maybe that helps?
It's an interesting point you raised.
[Edited on 24-2-2015 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
From Wikipedia http://en.wikipedia.org/wiki/Standard_electrode_potential_(d...
H2O2 as a reducing agent
H2O2 --> O2 + 2H+ + 2e- -0.70 volts
H2O2 as an oxidising agent
H2O2 + 2H+ + 2e- --> 2H2O 1.78 volts
Now what?
Why did my bromide to bromine oxidation work (-1.066V) and my chloride to chlorine not work (-1.36V).
Both were highly acidic solutions with a feisty amount of conc H2SO4 added to 6% H2O2.
What am I missing?
@blogfast25
[I follow deltaH's reasoning on the H+ equilibrium -- that is that the first reaction I have written is favoured in alkaline conditions and the second
in acidic. I will also concede that you probably have empirical evidence on your side with Cr and Ce. But I don't understand why this should be the
case. And I also fail to understand why Br and Cl should behave so differently. Are you saying I should have dumped some sodium hydroxide in there
instead of sulfuric acid? I don't get that. The reaction needs the H+. Tell me how confused I am. It wouldn't be the first time with redox (this
week).]
Edit
[tried to make] link work. [That's just annoying!]
And I see trhat metallus and blogfast have continued discussion in the cross-post.
[Edited on 24-2-2015 by j_sum1]
[Edited on 24-2-2015 by j_sum1]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
J_sum1:
I haven't go time to address your entire post right now but where you write:
| Quote: | | And I also fail to understand why Br and Cl should behave so differently |
That one's easy: bromide is easier to oxidise to bromine than chloride to chlorine, see the relevant oxidation potentials, which I don't have at my
fingertips right now (I prefer my CRC to Wiki for that kind of data).
BUT, from memory, H2O2 SHOULD be able to oxidise chloride to chlorine, though. IF memory serves me right... (note caveat, please)
My point about the oxidation/reduction of Cr(III) with H2O2 you'll also find in textbooks. I have one that explains what I observed and how I explain
it too.
[Edited on 24-2-2015 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That's alright. Take your time. I have other things to do too.
I agree: Chlorine should be possible. Both reactions are on the same side of the magic 1.78V figure. That's why I jumped at a kinetic explanation.
In practice, no appreciable amount of Cl2 was produced in a time frame of a few minutes. Throwing some chlorate in on top of the H2O2 got the job
done quickly enough that the demo wasn't a disaster.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | That's alright. Take your time. I have other things to do too.
I agree: Chlorine should be possible. Both reactions are on the same side of the magic 1.78V figure. That's why I jumped at a kinetic explanation.
In practice, no appreciable amount of Cl2 was produced in a time frame of a few minutes. |
I suggest to repeat your chloride to chlorine with H2O2 oxidation experiment. It should indeed work. Take close notes of what you do.
[Edited on 24-2-2015 by blogfast25]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Chlorine should be generated from the Cl- and acidified peroxide; the Ecell for this is +0.40 V (cf. 0.69 V for the analogous oxidation of Br-). This
only applies to standard conditions, and it may be worthwhile to account for differences in concentrations by using the Nernst equation.
As for the comments on the redox chemistry of hydrogen peroxide, please be aware that there are four half equations involved.
Under acidic conditions:
H2O2 + 2H+ + 2e- => 2H2O (oxidant, +1.776 V)
O2+ 2H+ + 2e- => H2O2 (reductant, +0.695 V)
Under basic conditions:
O2 + 2H2O + 2e- => H2O2 + 2OH- (reductant, +0.146 V)
HO2- + H2O + 2e- => 3OH- (oxidant, +0.878 V)
That last equation may be written more familiarly as H2O2 + 2e- => 2OH-
I guess the only way to determine whether hydrogen peroxide acts as an oxidant or reductant under given conditions would be to calculate delta G =
-nFE for the two different scenarios (in acid or base, as appropriate) and see which one is more favourable.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | Under acidic conditions:
H2O2 + 2H+ + 2e- => 2H2O (oxidant, +1.776 V)
O2+ 2H+ + 2e- => H2O2 (reductant, +0.695 V)
Under basic conditions:
O2 + 2H2O + 2e- => H2O2 + 2OH- (reductant, +0.146 V)
HO2- + H2O + 2e- => 3OH- (oxidant, +0.878 V)
That last equation may be written more familiarly as H2O2 + 2e- => 2OH-
I guess the only way to determine whether hydrogen peroxide acts as an oxidant or reductant under given conditions would be to calculate delta G =
-nFE for the two different scenarios (in acid or base, as appropriate) and see which one is more favourable.
|
Already done in a sense, no? Assume the Eox of the species to be oxidised then the cell potential for the oxidation with H2O2 becomes:
E = 1.776 + Eox in acid conditions and E = 0.876 + Eox in basic conditions. So acid conditions always trump (no matter the value of Eox, assuming E
< 0 of course) and yet Cr(III) is NOT oxidised to Cr(IV) acid conditions but it is in alkaline ones.
Possible causes may be that Cr(III) is complexed strongly and that in acid conditions dichromate (not chromate) would form?
[Edited on 24-2-2015 by blogfast25]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its just an acid/base dependant redox reaction:
In acid:
Cr2O7 2- + 14H+ + 6e- => 2Cr 3+ + 7H2O +1.33 V
Whilst in base:
CrO4 2- + 4H2O + 3e- => Cr(OH)3 + 5OH- -0.13 V
Coupled with the appropriate H2O2 half equations above, I'm sure you'll find everything is kosher.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep. That explains it.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Nice to know that my understanding was not waay off. (Unlike my previous gaffe!) I can still read a table of reduction potentials and make sense of it.
I would like to know a bit more about Cl2 production and Br2 production and H2O2 as an oxidant. Let me give details of what I did.
This was done as a demo -- outside on a scale to be viewable by 60 people. Amounts of NaCl and NaBr were calculated sufficient to make just over 1L
of gas/vapour at STP. (Gasses bubbled through a bucket of NaOH for safety. Windy day, audience upwind.)
Reaction was done in half litre conical flasks which should have been sufficient to allow visibility of gas to spectators.
6% H2O2 was used -- none stronger available at the moment. 20% excess calculated.
Acid was conc H2SO4, 50% excess added to the peroxide so both could be dumped into the flask together.
NaCl did show some bubbling -- faintly. I could not be certain that this was indeed Cl2. I always suspect O2 when I am dealing with peroxide. In
any case, it was insufficient to be visible at a distance. An attempt at the same thing the previous day at test tube scale also produced some minor
bubbling. I didn't smell it. There probably wasn't enough acid in that one so not a complete test. I scaled up to flask scale merely trusting the
redox potentials.
I have used KClO3 and KMnO4to oxidise Cl- to Cl2 before. Rather effective. My question is why H2O2 is so much less effective. Reduction potentials
suggest it should be more effective.
Cl2 --> Cl- (deltaV=1.36)
CLO3- --> Cl2 (deltaV=1.49)
MnO4- --> Mn2+ (deltaV= 1.51)
H2O2 --> H2O (deltaV=1.78)
My thoughts again turn to kinetics. Faint bubbling indicates that there is enough energy to drive the reaction, albeit slowly.
Is there a high activation energy at play here? How much does the dissociation of H2O2 come into play? Is the relatively low concentration of
peroxide a factor?
[edit -- get the reaction direction correct you idiot]
[Edited on 25-2-2015 by j_sum1]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
j_sum1:
Personally I have no explanation. I assume, without verification, that your calcs are correct.
How did you expect to produce 1 l of bromine at RT though?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Even if incorrect the same conditions were in place for Br and Cl.
1L of Br if it converted to vapour (which a lot of it did). IOW, equivalent molar quantities of Br2 and Cl2: 0.05 moles of each.
RT in Australia can be reasonably warm. About 30°C yesterday which is enough to make Br2 quite volatile.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
So the Br generation worked?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
My students are right here they will tell you what they saw. But yes, it worked.
| Quote: |
Hey, how you doing (laughs)
It went red -- very scientific terms. What I will be like one day. (I'm like a minus international hazard. I'm going to shut up now.)
Red brown vapours went through the tube and bubbled into the bucket.
|
Welcome to our class Blogfast 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, it remains a mystery why the Cl generation failed. I wish I could try it myself but health problems make it impossible, for now.
Try HCl + H2O2 in a test tube?
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Strange, based on my calculations, and on the water present and the temperature, only 7% should have dissolved assuming chlorine's solubility in a
sodium sulfate solution is the same as water - my guess is its slightly more soluble.
Since the container was full of air, and chlorine's color is very faint, I'm not too surprised it wasn't noticeable.
Even relatively pure chlorine is quite faint, but a 500 mL flask should show it.
Are you sure you didn't add more water?
This is a complete long shot, and by no means the only or best explanation but perhaps it is oxygen according to these reactions:
Cl2 + H2O --> HOCl (aq) + HCl (aq); HOCl + H2O2 --> H2O + HCl (aq) + O2
I doubt these reactions can happen fast enough, and why would they happen with chlorine but not bromine?
[Edited on 25-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
| Pages:
1
2 |