Astroboy
Harmless

Posts: 4
Registered: 10-4-2003
Member Is Offline
Mood: No Mood
|
|
Additive to Hydrogen Peroxide for rocket engines
To the audience of Energetic Materials:
This is my first topic placement on this forum. I'd like to firstly congratulate all of you for carrying such a professional and interesting
site, which I've been visiting for some time.
I decided to join the group, because I now believe that some of you might have the answer to my problem.
I run a small shop designing rocket engines and catalyst for amateur rocketry. My specialty is catalyst for hydrogen peroxide (H2O2) decomposition.
H2O2 as you may know, has been used since the 1920's in torpedo engine O2 source, V2 rocket turbine drive, Redstone missile, Lunar landing module
trainer and many reaction control rockets in satellites (Telstar) and early space rockets (Gemini). All of these applications used H2O2
concentrations from 70wt.% to up to 98wt.%.
Now that I set the mood properly, my reason for addressing you is to seek help in the search for an additive to ioncrease the decomposition energy of
H2O2 of concentrations of 50wt.% to 65wt.%. The reason being that, only two companies supply 90wt.% peroxide (FMC and Degussa) and they do not make
it available to private or small companies, only to military or NASA/ESA like agencies.
The performance of 90wt.% H2O2 is such that it can be safely used without a fuel (hydrocarbon or other), in a form known as "monopropellant"
or monergol mode.
Silver mesh screens are used to decompose catalytically the peroxide into water + O2 + heat. The adiabatic decomposition temperature of 90wt.% is
740C vaporizing the water into superheated steam. Below 70wt.% (233C) there is enough water in the solution to quench the decomposition reaction and
the thrust is dramatically diminished.
What I am looking for is for an organic or inorganic additive to 50wt.% peroxide solutions (easily available from several sources for pulp and bleach
industry at U$S 1.50/kilo) so it would have enough energy to simulate the performance of 90wt.% peroxide.
In the past alcohols have been added with sad outcome. Above 6 to 9wt.% of ethylic or methylic alcohols with H2O2 forms unstable ternary mixtures that
are explosive. Lots of data on this by NASA, FMC, Degussa.
I am looking into the possibility of adding Hydroxyl Nitro Urea (CH3N3O4, F.W.-121) or hydrazine nitrate (acidic) and see if I can boost the 50wt.% to
90wt.% performance. BTW, 90wt.% peroxide has a decomposition products volume ratio of 5,000 (for every liter of peroxide decomposed, 5,000 liters of
gas is produced, oxygen plus superheated steam).
Well enough ranting. Can anyone bless me with suggestions on how I might achieve my goals.
Sincerely,
Astroboy
\"Any time lost uphill, can never be recovered downhill.\"
J. M. Fangio - Five times F1 World Champion
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
50% H2O2 by weight means 1000g contain 500g H2O (27,77 mole) and 500g H2O2 (14,70 mole)!
14,7 mole of H2O2 will free 7,35 mole O2 (235g)!
The admixion of NH2-NH2 (82%) is roughly 100% NH2-NH3OH (comercially available);it is stable but might be hypergolic in contact with H2O2!So better
keep the two separated and injected in the reaction room!
NH2-NH3OH + O2 --> N2(g) + 3H2O(g) + heat
1kg of 100% NH2-NH3OH is thus 20 mole/kg!
To use properly the O2 available in the 50% H2O2 you will need 2,72 kg H2O2 per kg NH2-NH2 82%!
Thus final equation is:
3H2O + 2H2O2 + NH2-NH2 --> N2(g) + 7H2O(g)
You have to gather standard heat of burning of NH2-NH2; standard heat of decomposition of 2H2O2 and use that energy to heat up 7 H2O and N2(g); then
you will know the final T obtained and you would be able to estimate trust or kilo ratio)!
The use of NH2-NH3ONO2 has the problem it is solid fuel and has a positive OB!
The use of "Hydroxyl Nitro Urea (CH3N3O4, F.W.-121)" should lead to even more troubles!
NH2-CO-NH-NO2 is nitrourea (CH3N3O3) I don't see where the OH would go and what it would do to help stability???
I'll keep searching for infos!And results!

PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Astroboy
Harmless

Posts: 4
Registered: 10-4-2003
Member Is Offline
Mood: No Mood
|
|
more on Hydrogen Peroxide impetus improver
Dear PHILOU:
Thank you very much for replying to my post. Yes I do not want to mix hydrazine with H2O2 as they are hypergolic. In fact I do not want to make
hypergolic mixtures with my 50wt.%.
What I am wishing for is an additive to 50wt.% peroxide that would be stable with the peroxide until it is time to decompose it in the thrust chamber,
by contacting a catalyst, most typically silver mesh screens.
For example in the 1960 FMC experiemented with a mixture of 67.8wt.% H2O2 (100wt.%) + 6.8wt.% Diethylene Glycol (C4-H10-O3) + 25.4wt.% H2O. This
mixture with a density of 1.309gm/cc and a BP=225F had a specific Impulse (Isp) equal AND greater than 100wt.% H2O2 at 1ATM exhaust pressure.
The problem with this mixture is that I need to start with 67.8wt.% of 100wt.% H2O2 and, again that is hard or laboriously done (not to mention
risky).
The ideal additive would be one that could be used alone or in conjuction with other additives into starting peroxide solutions of 50wt.%
Astroboy
------------------------------------------------------
\"Any time lost uphill, can never be recovered downhill.\"
J. M. Fangio - Five times F1 World Champion
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
suggestion from Mr. Anonymous
| Quote: | | What about adding propylene oxide, and / or nitromethane to the percentage of its solubility in the mixture? Such a mixture might work, if everything
in the mixture is unreactive with each other. |
|
|
|
Astroboy
Harmless

Posts: 4
Registered: 10-4-2003
Member Is Offline
Mood: No Mood
|
|
Propylene oxide??
| Quote: | Originally posted by Polverone
| Quote: | What about adding propylene oxide, and / or nitromethane to the percentage of its solubility in the mixture?
NITROMETHANE I KNOW IT MIGHT NOT WORK FROM SOME TEST DONE IN THE 60'S, BUT PROPYLENE OXIDE? I'LL HAVE TO LOOK INTO IT. SOLUBILITY AND
STABILITY IS THE QUESTION.
THANKS,
ASTROBOY
Such a mixture might work, if everything in the mixture is unreactive with each other. |
|
\"Any time lost uphill, can never be recovered downhill.\"
J. M. Fangio - Five times F1 World Champion
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Virtually any reducing agent you add in quantity that will significantly affect performance will render the mixture detonatable, as you have a mixture
of oxidising agent and reducing agent mixed at the molecular level and this is usually all that is required.
Epoxides are strained and participate readily in ring opening reactions, probably resulting in a mixture of glycols and hydroperoxides. I'm not
sure how fast in the slightly acidic peroxide solution this would be, but its not anything Id want to try.
In small amounts concentration of the peroxide solution is possible, through without resorting to distillation loss will significant and production
will be very slow, whatever method you choose, evporation, freezing out...
If you really want to go this route, having people suggest additives there is no data for, will only cause accidents. Pick a known mixture, study the
detonation curve, pick a distance far enough away from it to be considered safe, then attempt to detonate the mixture deliberatly under different
conditions. The curves last I looked suggested that pure peroxide solution can be detonated and this is not I think the case, the amount of oxidiser
needed is tiny the higher the concentration you go though. The vapour above 90% peroxide will detonate without anything added IIRC producing higher
than the 78%? concentration limit needed in the gas phase.
If you are registered to make the rockets, have the requisite health and safety stuff, then you should be able to find someone to sell you 70%+
peroxide which should be better performing than any safe mixture with 50% you could make would be. If you are after a bit of extra power this should
be enough, but if you are after all out performance 90% peroxide monopropellent would not be enough either.
[Edited on 15-4-2003 by Marvin]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Never too late to add to the knowledge base
Invaluable propellant data resource : http://www.astronautix.com/props/index.htm
Related threads in our forum _
http://www.sciencemadness.org/talk/viewthread.php?tid=8746&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=6108
http://www.sciencemadness.org/talk/viewthread.php?tid=1151
| Quote: | Originally posted by Astroboy
What I am looking for is for an organic or inorganic additive to 50wt.% peroxide solutions . . .
so it would have enough energy to simulate the performance of 90wt.% peroxide. |
From page 71 of " Liquid Rocket propellants "
http://stinet.dtic.mil/cgi-bin/GetTRDoc?AD=0405726&Locat...
| Quote: |
The power factors of monopropellants based on hydrogen
peroxide can also be increased by preparing mixtures containing,
in addition to peroxide and water, such fuel components as ethyl
alcohol, glycerine, acetone, and others. Thus, by Introducing
8% ethyl alcohol in a 50%e aqueous solution of hydrogen peroxide,
we can obtain a monopropellant with a combustion temperature near
800ºC. Such a mixture is more powerfUl than hydrogen
peroxide of 80% strength and Is safer to handle. |
This is not stoichiometric , emphasis is for a safe mixture for gas production
See the phase diagram bottom of this post _
By the formulation given in the quote :
( 50 % strength H2O2 is 46% content with 46%
water ) + 8 % ethanol
( mol weight of C2H5OH = 46 ) X coeficient 1224 = 56304 weight
( mol weight of H2O2 = 34 ) X coeficient 9522 = 323748 weight
( mol weight of H2O = 18 ) X coeficient 17986 = 323748 weight
Total Weight = 703800
C2H5OH = ( 56304 / 703800 ) = .08
H2O2 = (323748 / 703800 ) = .46
H2O = (323748 / 703800 ) = .46
Balanced equation in lowest terms
1224 C2H5OH + 9522 H2O2 + 17986 H2O => 2448 CO2 + 31180 H2O + 1089 O2
28732 mols of fuel yields 34717 mols of gas
1.208 times volume increase not accounting the calorimetric expansion.
* Note I omitted to mention that only ethanol used for fuel is anhydrous.
Alchohol which may be drunk is up to 190 proof ~ 95% with the balance
water , and is subject to federal liquor exise tax. Hmm I wonder if sugar
could be safely be used as a fuel additive.
_________________________________
Much more interesting on page 64 it mentions an oxidizer blend of
( 90 % strength H2O2 is 54% content with 6 %
water ) + 40% of NH4NO3
This must be a forceful monopropellant on its own without any fuel additive.
( that would make it a detonable mixture , a very bad rocket fuel )
As before:
( mol weight of NH4NO3 = 80 ) X coeficient 102 = 8160 weight
( mol weight of H2O2 = 34 ) X coeficient 324 = 11016 weight
( mol weight of H2O = 18 ) X coeficient 68 = 1224 weight
Total Weight = 10200
NH4NO3 = ( 8160 / 20400 ) = .4
H2O2 = ( 11016 / 20400 ) = .54 ,
H2O = ( 1224 / 20400 ) = .06
Balanced equation in lowest terms
102 NH4NO3 + 324 H2O2 + 68 H2O + => 596 H2O + 204 N2 + 213 O2
494 mols of fuel yields 1013 mols of gas
Hmm 2.05 times volume increase not accounting the calorimetric expansion.
__________________________________
Explosive and Combustion Properties of H2O2
Pure H2O2 is readily detonable and its heat of explosion is given as 24.6 kcal/mole.
It is claimed that aqueous solutions of less than 94% H202 will decompose explosively
if catalyzed, but will not detonate. More recent studies, however, show that 86% H202
at 50° C or higher and contained in 1.61 inch ID Al tubes, will detonate at 5600 m/sec
when strongly boostered, but not at smaller diameters. A 90.7% solution detonates at
‘5500 to 6000 m/sec even at 0.5 inch ID and 25° C. The tube size which the 90.7%
solution will propagate detonation increases with increasing temperature.
In view of the above , 80 % strength in combination with NH4NO3 would
seem to be the highest " safe " concentration , this is only a guess though.
Without actual test data , increased sensitization by the presence of bubbles
is a definite risk to consider.
U P D A T E -
W R O N G ! ! significant explosion risk exists with 70 % concentration
comprising as low as 20 % of a mixture. See - Condensed Phase Explosibility http://web.archive.org/web/20030426074004/www.ee.surrey.ac.u...
Phase Diagram for Mixtures of Hydrogen Peroxide and Ethanol
[Edited on 9-9-2007 by franklyn]
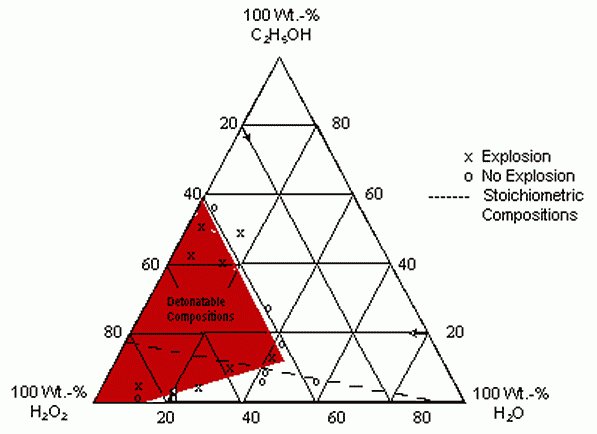
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Those terrorists who were arrested in Germany recently, when in the final stages of making bombs using H2O2 (probably with acetone to make acetone
peroxide, or else with flour), certainly were not going to use their 700 Kg of the stuff for rocket engines!
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
H2O2
The explosives property of 90%+ H2O2 are impressive. In the early 1980s I was fortunate
enough to see a rocket car, named Vanishing Point, use 90% H2O2 with a silver nitrate
catalyst. 20 ounces of this fuel were enough to propel the car to 365 MPH in about 4.5
seconds. It looked like a giant steam cloud coming out of the nozzle and was a lot
quieter than the jet cars.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Boomer
Hazard to Others
  
Posts: 190
Registered: 11-11-2005
Member Is Offline
Mood: No Mood
|
|
"probably with acetone to make acetone peroxide, or else with flour"
The concentration was reported as 35%, this looks like they were planning bulk TATP production....
Probably in multiple batches for multiple targets. Just imagining pouring several 50-pound sacks of dry TATP into barrels (aka Tim M.V.) makes me
think it would most likely go up from static, unless done on a *really* misty/rainy day.
Why flour? To get a detonable mix, even 50% is on the low side. Possible with fine Al, or liquid organics, but not flour. Am I totally off?
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Flour ?
Maybe they're looking for a more stable and transportable mix as we all know how unstable
AP is. Somehow, the flour mix doesn't seem as efficient and probably harder to detonate.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This is a rocketry related thread , why has it abruptly changed into yet another
TATP terrorist diatribe. JEEeez 
Please See _
http://www.sciencemadness.org/talk/viewthread.php?tid=9118
.
|
|
|