Cappy
Hazard to Self
 
Posts: 92
Registered: 27-3-2003
Member Is Offline
Mood: No Mood
|
|
N3, a longshot.
Would it be possible to make an N3 molecule like this? I've never heard anything about N3, but I thought it might be a good explosive if it were
stable enough to store for short periods.
2N3 --> 3N2
[Edited on 4/3/2003 by Cappy]
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
No, there would be to many unpaired electrons. Next even if they formed a double bond and made a positve cation i think the strain on the 60* bonds
wouldnt hold. The linear structure is an easyer route for the azide molecule. This is really not based on any info just an educated guess by me.
CTR
|
|
|
Cappy
Hazard to Self
 
Posts: 92
Registered: 27-3-2003
Member Is Offline
Mood: No Mood
|
|
Silly me! What was I thinking!?
Okay, how about this:
| Code: | N
/ | \
N--|--N
\N/ |
I know this graphic is bad, but I was going for tetrahedral N4.
Any ideas on the boiling point for such a molecule? My guess is pretty darn low. This makes high density difficult, but at least you get two moles of
nitrogen triple-bonds per mole of N4.
N4 --> 2N2
[Edited on 4/3/2003 by Cappy]
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
The bonds would be stressed far to much for that to exist. IMHO
CTR
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
if you look at the other post (Nitro Fullerene) u'll have all answer (message to other that didn't saw it)
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The best molecules theorized that contain only N as element are:
*aromatic N6 based on C6H6 squelton!
*a kind of fullerene N20 made only of pentarings where N has 109° bonding angle!
You need to reduce as much bonding stress and inhomogeneity in bond lenghts!So all N have to be equivalent and symetry is an asset for stability!

PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Cappy
Hazard to Self
 
Posts: 92
Registered: 27-3-2003
Member Is Offline
Mood: No Mood
|
|
I'm just going to venture a guess. Is this because the molecule is only as strong as it's weakest link? If the bonds are assymetrical, some
of the bonds may be stronger than if they were symetrical, but some would be weaker, right? Also, the weaker assymetrical bonds would increase
sensitivity, while the stronger bonds would increase the energy required to fully decompose the molecule (resulting in less net energy released).
I'm just sorta guessing, but is my hypothesis true?
[Edited on 4/7/2003 by Cappy]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Almost right!
When you have R-N-N=N-R sequence; the = link is stronger and shorter than the other NN links; thus cleavage will be favourised as:
R-N° and °N=N-R or as R-N-N=N° and °R!
The resulting R-N=N° and R-N-N=N° soon looses N#N (N2) with release of energy!
R-N=N° --> R° + N2 + En
R-N-N=N° --> R-N° + N2 + En
This is easily understandable if you consider vibrational molecular model!
Those compounds with unequivalent N linkings are weakest links generators and are thus source of unstability!
The presence of N=N links favourise the freeing of N#N (N2) gas what release a lot of free energy (increases entropy).
As a simple evidence for this compare sensitivity and existancivity of
HCl.NH2-NH-C6H2(NO2)3 (trinitrophenylhydrazine hydrochloride)
vs
Cl-N=N-C6H2(NO2)3 (trinitrophenylazonium chloride)
The first one is quite stable to heat and shock while the second is very sensitive and doesn't exist long even in the cold due to spontaneous
decomposition!
A little calculation will help here!
R2N-NR2 + 90 kcal --> 2R2N(g)
N2(g) + 226 kcal --> 2 N(g)
So
10 N2 + 2260 kcal --> 20 N
20 N --> N20(g) + (90kcal* 30 links = 2700 kcal)
Of course
N20 (l or s) + Energy --> N20 (g); thus value is superior!
N20(g) should have an inner energy (endothermic) of approx 2700-2260kcal = 440 kcal/mol = 1843,6 kJ/mol =
1843,6 kJ/280g = 6584,28 kJ/kg
This not counting the heat of vapourisation!

PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Solid Allotropes of Nitrogen
Solid nitrogen was still stable after the pressure was reduced to normal, but only when liquid nitrogen was used to maintain a low temperature.
"An allotropic form of nitrogen was synthesized directly from molecular nitrogen at temperatures exceeding 2500K and pressures above 110 GPa [Eremets,
Gavriliuk et al., 2004]. This phase can be quenched to ambient pressure due to the large hysteresis of the material, but only at low temperatures,
precluding performance testing of the material [Eremets, Gavriliuk et al., 2004]."
There is also a "black phase" of solid nitrogen, which is stable at room temperature, but only under extreme pressure, or alternatively, stable at
ambient pressure, but only when cryogenic temperatures are maintained.
" a new, dark, apparently non-molecular phase has been recently found above 180 GPa at 80 K [3] and then at room and elevated temperatures. Some
properties of this black phase are close to that predicted for the polymeric nitrogen; that is, the value equilibrium pressure (about 100 GPa), and a
huge hysteresis enabling the black phase to be recovered at ambient pressure and low temperatures."
Apparently this solid polymeric forms of nitrogen.
A.F. Goncharov et al Phys. Rev. Lett. 85, 1262*1265 (2000);
E. Gregoryanz et al Phys. Rev. B 64, 052103 (2001), 224108 (2002).
These allotropes are completely different from plain solid nitrogen, which freezes at -210.01 degC.
Frozen nitrogen (still composed of individual N2 molecules) is a solid with a density of 1.026 g/cm3 at -252 deg C.
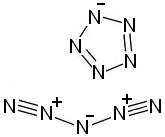
All-nitrogen salt? N10
Researchers are currently trying to make vissible quantities of a pentazolate salt, from which they hope that an all-nitrogen ionic salt will be
possible. An allotrope of elemental boron, containing clusters of positively and negatively charged all-boron polyatomic ions, is already known to
exist. (see references at bottom of post)
They are trying to make the N5(-) ion, but so far have not been successful. The reason that this ion is so desirable is that they calculate that it
would NOT be oxidized by the N5(+), which already has been prepared. Unfortunately azide ions N3(-) are oxidized by N5(+), and it only generated
nitrogen gas. So they are desperately trying to make the N5(-) so they can see if an ionic allotrope of nitrogen is possible! Boron is already known
to have an ionic allotrope, for example, where the boron atoms exist is cation and anion clusters.
If they do succeed in making the N5(+) N5(-) salt, it would be interesting to find out how it compares to other explosives, whether packing on
nitrogen atoms is the best strategy for an ideal molecule. The calculations are that the all-nitrogen salt would be 2-3 times more energetic than RDX.
Preparation of Pentazolate Anion N5[-]
Zinc bromide can be used to catalyze the reaction of sodium azide with nitriles (such as methyl cyanide) in hot water to form tetrazoles.
1,2,3-triazoles are surprisingly stable. Tetrazoles are also fairly stable and only moderately sensitive to detonation, although they can be
hydrolyzed into much more dangerous azido compounds in reactions. Plain tetrazole melts at 158degC, and decomposes over 180degC.
Aryl diazonium salts can be reacted with azide to form aryl pentazolates. An example of such an aryl diazonium salt is 4-Dimethylaminophenyl diazonium
nitrate (CH3)2N[C6H4]N(+)N NO3(-) , which can be formed by reacting 4-Dimethylamino-analine (where the two nitrogen atoms are on opposite ends of
the benzene ring, with one of the nitrogens having two methyl groups on it, and the other two hydrogens on it) with dilute nitric acid and sodium
nitrite. The 4-Dimethylaminophenylpentazole thus formed is decomposes after several hours at room temperature, or immediately at 50degC, although
decomposition is negligible at cryogenic temperatures.
Removing the pentazolate ring to form the N5(-) ion has not yet been done. One idea would be add three nitro groups to the benzene ring using acetic
anhydride and copper(II)nitrate, then hydrolyze the tetrazolate group off by reacting with tetramethylammonium hydroxide.
The pentazolate ion seems to decompose on reaction with ammonium ions.
Pentazolate derivitives can be somewhat stabilized by forming a complex with zinc ions.
Pentazenium Cation N5[+]
http://en.wikipedia.org/wiki/Pentazenium
references for ionic boron allotrope:
Oganov et al. Ionic high-pressure form of elemental boron. Nature, 2009
Solozhenko VL, Kurakevych OO & Oganov AR. On the hardness of a new boron phase, orthorhombic
Journal of Superhard Materials, 2008; 30: 428-429
http://docs.google.com/viewer?a=v&q=cache:f56F4Vwi2IQJ:w...
[Edited on 20-9-2011 by AndersHoveland]
|
|
|
eyeofjake
Harmless

Posts: 6
Registered: 2-1-2012
Member Is Offline
Mood: No Mood
|
|
A cyclo-nitrogen ring......imagine the instability if possible. Just thinking about it in a negative way would set it off. A new level of sensitivity:
insensitive to psychic energy.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Direct download of paper above in google
All Nitrogen or High Nitrogen Compounds as High Energy Density Materials
www.dtic.mil/dtic/tr/fulltext/u2/a438891.pdf
.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by Cappy  |
Would it be possible to make an N3 molecule like this? I've never heard anything about N3, but I thought it might be a good explosive if it were
stable enough to store for short periods.
2N3 --> 3N2
[Edited on 4/3/2003 by Cappy] |
Maybe, just maybe; you might be able to use N3 as a intermediate in a reaction, thus reaping some of the energy--or at-least something different.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The N3 molecule would be a radical. Two N3 molecules would immediately react with eachother as soon as they collided, decomposing into three N2
molecules. The N3 molecules would also be very unstable because of the bond strain (being a triangular ring), and also because of the lone pair
repulsion.
You might do some research into the oxidation of sodium azide.
The basic answer is no, it is not possible to isolate N3 as a pure compound, although it could theoretically be possible to make N3 molecules and trap
them into a crystal (of some other compound) at low temperatures.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Why are we looking so much at nitrogen? There are metals like Mg that are kinda energetic. And oxidizers with these O-F bonds that should be also
energetic. In rocketary if nitrogen is involved, the impulse isn't to high I think.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by AndersHoveland  | The N3 molecule would be a radical. Two N3 molecules would immediately react with eachother as soon as they collided, decomposing into three N2
molecules. The N3 molecules would also be very unstable because of the bond strain (being a triangular ring), and also because of the lone pair
repulsion.
You might do some research into the oxidation of sodium azide.
The basic answer is no, it is not possible to isolate N3 as a pure compound, although it could theoretically be possible to make N3 molecules and trap
them into a crystal (of some other compound) at low temperatures. |
Now that...would be cool. Any Ideas as to how one would go about doing that, and what crystal to trap them in?
|
|
|