CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Bras ,batteries ,benzene and something in between
for quite sometime now ,many questions have been bothering me. They span across all fields ,from organic chemistry ,to reagents to lab techniques for
carrying out certain reactions
making a thread for each of them would be a waste of this forum's valuable space which could be used for better posts
so i will arrange my questions according to the field they belong so that if they are interesting enough,they can be moved to the appropriate sections
1.Reagents
I recently had a chance to work with raney nickel ,and i have completely fallen in love with it 
the sheer reducing power at room temperature and atmospheric pressure without the need for external hydrogen makes other expensive reducing agents
look like babies
what i was looking for is an OTC source of nickel ,which could be alloyed with aluminum (many people have tried that) or the alloy directly (Ni:Al
1:1) so that i could treat it with sodium hydroxide according to the orsyn page
but i have failed to find such an OTC source
what i have found though is that there is an OTC source of a nickel titanium alloy (the Ni:Ti ratio is also nearly 1:1) called nitinol
http://en.wikipedia.org/wiki/Nickel_titanium#Applications
read the last application 
so my question is -could nitinol be used instead of Ni:Al to make raney or atleast a weaker version of it
(although you would need a lot of underwire  ) )
also ,i found the preparation of Raney nickel of W-2 and W-6 grade .could someone please give the links for making the other grades (raney nickel is
available from W-1 to W-7 ) or atleast tell me how to get them 
i have tried googling,but nothing has turned up
i also searched in the Orsyn website ,but i didnt find anything
2.lead tetraacetate
lead tetra acetate is an amazing oxidising agent ,but it is also an alternative to using silver for the hunsdiecker reaction
http://en.wikipedia.org/wiki/Hunsdiecker_reaction
like raney ,i have been hunting for an OTC source of red lead
i found an old thread in which someone tried to make it
http://www.sciencemadness.org/talk/viewthread.php?tid=3498
they didnt tell the source for the red lead though
could red lead be made by heating spongy lead (from batteries)
as red P can be made from white P by heating
http://en.wikipedia.org/wiki/Allotropes_of_phosphorus#Red_ph...
and tin transforming to other allotropes at low temperature
http://en.wikipedia.org/wiki/Tin_pest
or is there a better source for red lead ?
2.Organic chemistry
1. I came across this reaction a few days back
http://www.orgsyn.org/demo.aspx?prep=cv1p0341
could the same be done with acetaldehyde to get benzene ?
(the yields are low though  ) )
2.i read about this reaction ,a long time ago
http://en.wikipedia.org/wiki/Bucherer_reaction
the wiki page says that it is only for napthols
but why cant this be done for phenols as well
in the reaction mechanism,the second benzene ring does not seem to take part ,or am I missing something 
3.Can HI/red P reduce COOH to methyl directly
more specifically ,suppose you had cyclobutane-1,1,3,3-tetracarboxylic acid
http://www.chemicalbook.com/ChemicalProductProperty_EN_CB124...
and you refluxed it with HI/Red P ,would all the COOH become CH3 ?
(actually if you reflux gem dicarboxylic acids in HCl ,it will get decarboxylated)
does the same thing happen for even HI/Red P ?
3.Utilizing Good rearrangements in sythesis
I was going through the synthesis of camphene from isoborneol
isoborneol http://www.sigmaaldrich.com/catalog/product/aldrich/i13901?l...
camphene http://en.wikipedia.org/wiki/Camphene
at a glance ,the synthesis appears quite hard 
but on observing carefully ,you can tell that it is a simple acid catalysed dehydration followed by a Wagner meerwein rearrangement
http://en.wikipedia.org/wiki/Wagner%E2%80%93Meerwein_rearran...
so my last and final question is -how do you train your brain to think about this types of rearrangements while doing a retrosynthesis ?
do you have to read any books dedicated to this topic (of using rearrangements in organic synthesis)
or are these discovered by accident while carrying out some other procedure ?
i would be very grateful if someone would reply to my questions
especially the last one 
[Edited on 9-12-2014 by CuReUS]
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
I believe red lead can be ordered from pottery supply websites (try http://www.seattlepotterysupply.com/). It say "not available", but you may be able to find it at other pottery supply websites.
|
|
|
Amos
International Hazard
    
Posts: 1408
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Red lead isn't an allotrope of elemental lead; It's lead(II,IV) oxide, which is immediately revealed on using a google search. It is sold as mentioned
above by ceramic supply companies and is often contained in anti-fouling paints used for boats. If you're having trouble producing red lead, why not
try one of the wikipedia methods and see what you get? I like the method that describes mixing solutions of potassium plumbate and lead(II) acetate.
The plumbate could be prepared by dissolving lead(IV) oxide in an alkali metal hydroxide. It's a long process getting there, though.
[Edited on 12-9-2014 by No Tears Only Dreams Now]
|
|
|
phlogiston
International Hazard
    
Posts: 1383
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
A source for nickel metal could be cast iron welding electrodes (99% nickel).
Nickel salts are used in electroplating solutions, and you can get the oxide at ceramic supply stores.
Otherwise, there are various threads on the forum on extracting it from coins or batteries.
How do bras fit in all this?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Amos
International Hazard
    
Posts: 1408
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
The nickel aluminium alloy he mentioned is used for the underwire in bras.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
I've got some odd looks in the past when buying things for their chemical content, but I think this one would take the cake.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Quote: Originally posted by Oscilllator  |
I've got some odd looks in the past when buying things for their chemical content, but I think this one would take the cake. |
It is not a nickel/aluminum alloy but nickel/titanium.
And sure, bras are OTC, but an underwire bra with nitinol wire is about $60, and you are going to get maybe 5 or 10g from it? Just buy some memory
wire on eBay. As a popular science gimmick, it is cheap and readily available.
Here is 50 feet of 0.7mm wire for $25. Or you could try thrift shop bras, but there is no guarantee that the wire is Nitinol.
Or here is a 5x200mm rod.
If you are looking for nickel metal:
304 stainless steel is 8-10% Ni.
US $0.05 coins are 25% nickel and 75% copper. Many other coins from various countries have nickel content.
AlNiCo magnets contain anywhere between 15 and 30% Ni.
Or you can buy the metal cheaply on eBay in good purity.
I have some info on your other questions too but I am busy and will post it later.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
nickel salts wont help ,i need the metal not the ions 
but if there is a good way to reduce the ions to the metal ,please post it here
maybe by forming an unstable complex,that decomposes on heating to give the metal (in this case Ni)
like Ni(CO)4 (but obviously not that one  ) )
i never said it was ,i just tried to make an analogy with other reactions,in which one element changes to another by changing temperature
obviously , the analogy made no sense whatsoever 
I am very sorry if I confused you
what I wanted to convey was whether red lead could be made by heating spongy lead(found in lead acid batteries) to high temperature in a crucible or
by using a blow torch.
Praxichys
what a fool i have been 
i should have checked about nitinol on ebay first
magicians use it for the payphone trick 
i would have to alloy nickel with aluminum ,that too in a 1:1 ratio to be able to use it for Raney ,if i only bought nickel in its pure form ,so that
was my last resort ,because i dont think that would be easy(or maybe i lack the skills )
after posting my last post ,i thought that nitinol could not be used as titanium would not react with NaOH( i thought )
but surprisingly ,it does 
http://chemindustry.ru/Sodium_Hydroxide.php
read the last two lines above the chemical equation for the reaction of Al with NaOH ( it is the 3rd equation in the page)
could someone explain why ?
[Edited on 9-12-2014 by CuReUS]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
In the uk you can sometimes get red lead in proper car spares shops (not halfords), or places they sell car bodywork stuff, I think its sometimes used
as a anti rust coating or something
Dont ask me, I only know enough to be dangerous
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
nickel salts wont help ,i need the metal not the ions 
but if there is a good way to reduce the ions to the metal ,please post it here |
Do note the oxide source.
As for reducing the ions to standard state, electroplating itself can do this, or a chemical alternative known as "electroless nickel plating." Not as
easy as purchasing the oxide for ceramics, but still available from OTC items.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
i dont require nickel anymore ,as nitinol is available so easily
i will follow the orsyn page for making raney nickel, but i will use the Ni:Ti alloy instead of the Ni:Al alloy
if this works ,this could be an OTC source to a very powerful reducing agent 
those who disagree should also note the fact that raney nickel is a "watched" reagent along with Pt/Pd black
also ,i was comparing the cost of raney nickel from different buyers ,and it looks very fishy indeed
one buyer is selling it for 14$/Kg (yurui chemicals co) whereas another is selling it for
5-100$ /g  (shanghai ruizheng chemical technology co) (shanghai ruizheng chemical technology co)
also i asked earlier if benzene could be made by reacting acetaldehyde with sulphuric acid
i remembered later that paraldehyde would form 
https://www.erowid.org/archive/rhodium/chemistry/acetaldehyd...
[Edited on 13-12-2014 by CuReUS]
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
I don't know about your specific example, but yes: COOH can be reduced to CH3 by the method you suggest in the first post. There are examples posted
as attachments on the forum, because I remember that is where I first heard of it.
To answer your last question in the first post, personally I spent a lot of time just looking at pictures from OrgSyn website. I downloaded them all
and went through them one by one, but quite quickly. There are some surprising reactions that are useful to know. Any that catch your interest as
useful you can put to one side to look up later. All procedures there are high yielding and reproduceable, so it is more useful to use that as a
source than scifinder or whatever people use these days, where a reaction may look useful, but you find it has a low yield on closer examination.
[Edited on 13-12-2014 by forgottenpassword]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
is there any other way to make ethane 1,2 dithiol instead of the wiki page method
http://en.wikipedia.org/wiki/1,2-Ethanedithiol
because although ethane 1,2 dichloride can be easily made(ethylene glycol+HCL reflux),I dont think NaHS will be OTC
also,i was trying a retrosynthesis of alpha pinene
http://en.wikipedia.org/wiki/Alpha-Pinene
and I decided to do acid dehydration on this molecule to get alpha pinene,but i cant figure out the reaction mechanism,so can someone help me 
[Edited on 31-12-2014 by CuReUS]
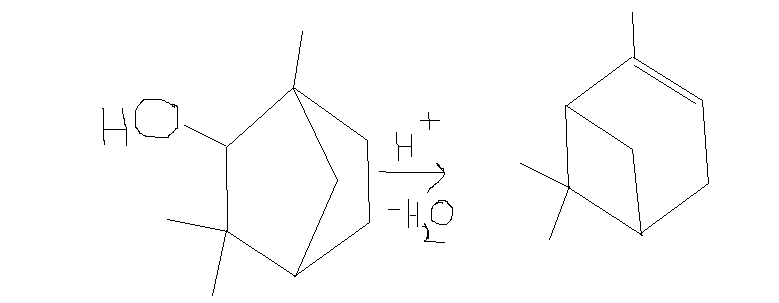
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
I'm assuming that this is a known reaction, because otherwise writing a plausible mechanism for it would be useless:
Remove H2O to give a secondary carbocation. Move a C-C bond to give the tertiary carbocation. Deprotonate your tertiary carbocation to give the
alkene. You can see which is the tertiary carbocation from the structure of the final product, so which C-C bond to break/make should be obvious. Make
a 3D model of the molecule if it is not clear to you why this bond moves.
You didn't click on the OrgSyn link in the references? There they use thiourea; as well as listing alternate methods in the discussion section. You
could make your own H2S (and thus NaSH) from FeS and dilute acid, I suppose, but it's a dangerous and smelly business. The thiourea route looks much
more agreeable.
[Edited on 31-12-2014 by forgottenpassword]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
the idea was based on the wagner meerwein rearrangement of isoborneol to camphene,I dont know whether this exists or not 
http://commons.wikimedia.org/wiki/File:Isoborneol2CampheneCo...
| Quote: | | Deprotonate your tertiary carbocation to give the alkene |
but wont the deprotronation take place such that the double bond forms outside the ring rather than inside ?
| Quote: | | You didn't click on the OrgSyn link in the references? There they use thiourea; as well as listing alternate methods in the discussion section. You
could make your own H2S (and thus NaSH) from FeS and dilute acid, I suppose, but it's a dangerous and smelly business. The thiourea route looks much
more agreeable |
I didn't consider the thiourea route because I dont think thiourea is OTC, is it?
but your idea of using FeS can be done.Iron and sulphur are both OTC(sulphur from gun shops ? ) and heating both of them will give FeS with which
ethane 1,2 dibromide can be reacted in presence of H2SO4 to get the ethane 1,2 dithiol 
[Edited on 1-1-2015 by CuReUS]
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
If you don't know that it is a reaction which actually occurs, then why is it part of your retrosynthesis? Writing a mechanism for it won't make the
reaction work in real life if it doesn't anyway! I thought that the whole point of retrosynthesis was to use KNOWN reactions to get from one chemical
to another?! Anyone can make something work with a pen and paper and a few arrows, but it's what actually happens that matters.
Yes, thiourea is easily availiable, and much prefereable to generating hydrogen sulphide.
[Edited on 1-1-2015 by forgottenpassword]
|
|
|
Megatron
Harmless

Posts: 2
Registered: 1-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  | Quote: Originally posted by phlogiston  |
nickel salts wont help ,i need the metal not the ions 
but if there is a good way to reduce the ions to the metal ,please
|
Make and warm a Chloride solution from your oxide/coins whatever,then precipitate a finely divided catalytic nickel, by addition of zinc powder. This
form of nickel has proven active enough to reduce various substrates in aqueous solution as is.
Papers discussing Zn/NiCl2 systems are posted on this board I'm sure. |
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by forgottenpassword  | | If you don't know that it is a reaction which actually occurs, then why is it part of your retrosynthesis? Writing a mechanism for it won't make the
reaction work in real life if it doesn't anyway! I thought that the whole point of retrosynthesis was to use KNOWN reactions to get from one chemical
to another?! |
no wonder I was having problems trying to figure out the mechanism.Before I was thinking that maybe I was doing something wrong in the mechanism but
now I realise that the wagner meerwein rearrangement cannot be applied to this substrate to get alpha pinene
| Quote: | | Yes, thiourea is easily availiable, and much prefereable to generating hydrogen sulphide |
that's amazing  please give me the source please give me the source
but why does the H2S have to be generated in-situ ,can't alcoholic FeS be reacted with ethane 1,2-dibromide to get the dithiol or is FeS
not ionic enough?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I am looking for an OTC source of isobutanol
http://en.wikipedia.org/wiki/Isobutanol
the wiki page says it is found in solvents,varnish remover etc,but I haven't been able to find it 
so I have come up with this route:
1.take valine and do strecker degradation using bleach
http://www.amazon.com/BulkSupplements-Pure-L-Valine-Powder-g...
http://en.wikipedia.org/wiki/Strecker_degradation
2.reduce the aldehyde formed to isobutanol using MPV reduction
http://en.wikipedia.org/wiki/Meerwein%E2%80%93Ponndorf%E2%80...
does anyone have any better ideas  ? ?
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I see lots of it on eBay. Do you live in the USA? You can get a whole gallon for $83 from Illinois, or a liter for $30 from PA.
I can personally vouch for the last seller, having purchased many things from him.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
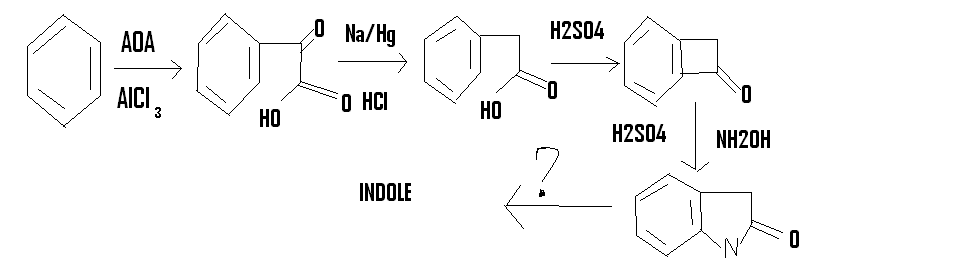
I have been fascinated with heterocyclic compounds for a long time
so when I saw fisher's indole synthesis,I decided to come up with my own
but I am unable to do the last step of converting the amide to indole.could someone help me?
AOA is acetic oxalic anhydride
http://en.wikipedia.org/wiki/Acetic_oxalic_anhydride
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I realize now that AOA will not react the way I want it to.then the only other way is using bromoacetic acid and doing a Friedel craft alkylation
followed by treatment with SOCl2 and then a F.C acylation to get the compound before the beckmann rearrangement step
also ,regarding my nitinol idea,I read a paper that says Ti is resistant to NaOH 
but the russian website claims that it reacts vigorously
some one please help .I am really very disappointed
Its actually very surprising because the reduction potential for Al is -1.66 V whereas for Ti it is -1.63 V
paper - http://www.parrinst.com/wp-content/uploads/downloads/2011/07...
read pg 17
| Quote: | after posting my last post ,i thought that nitinol could not be used as titanium would not react with NaOH( i thought )
but surprisingly ,it does 
http://chemindustry.ru/Sodium_Hydroxide.php
read the last two lines above the chemical equation for the reaction of Al with NaOH ( it is the 3rd equation in the page)
could someone explain why ?
|
so I guess I wont be seeing a reaction like this with nitinol 
https://www.youtube.com/watch?v=bhORLiiij3I
[Edited on 17-1-2015 by CuReUS]
|
|
|