BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
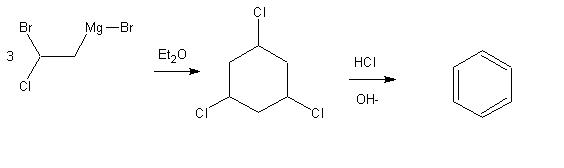
Crazy Benzene-synthesis
I was wondering if that could work:
.....................................Cl
......................................|
.....................................C
....Br......MgBr........&........&
......\....../.....Et2O.C.............C...-OH
3 Cl-C--C-H..=>..&.............&..=>.benzene
....../......\..............C.............C...-HCl
....H.......H............/...&......&....\
.........................Cl........C........Cl
&........C-C single-bond
I have not yet seen any reaction with a grignard that makes a cyclic compound so far...should this discourage me? 
[Edited on 11-3-2003 by BASF]
[Edited on 11-3-2003 by BASF]
[Edited on 11-3-2003 by BASF]
[Edited on 11-3-2003 by BASF]
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
NOOOOOOOO!
This is running me mad........argh
it was all ok till i posted it to the board
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
It is a *** art to draw structural formulas with ASCII, and when you post it to the board, all the gaps get messed up......
I hope you can still figure it out of the schematic with looking twice...
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
You might want to consider downloading "ISIS Draw" for diagramming organic reaction schemes. A google search will turn up a download. 
I weep at the sight of flaming acetic anhydride.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
or using [code][/code]
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Chemsketch is far superior if you ask me.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Anyway i'm trying to figure out what it's write and here my conclusion...
I can't tell if it's work but see by yourself
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Hey thanks.....
You´d only have to change -OH being on top of the arrow in the last step, reagents are always on top of reaction arrows and the leaving groups or
eliminated species are below, so then -HCl(the negative sign does not mean charge but that it is a leaving group in this case, while the -OH is the
counter-ion of Na+ in here).
Sorry for not writing OH-, which is from a structural point of view wrong, because the negative charge is on the oxygen in reality, but i have to
admit it would have been less misleading....
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
My pleasure, thanx for the info, and personnely i don't have much knowledge about grignard reagent, but i think it may work. I don't really
understand why do it need a Chlorine? (and also what would be the name of the grignard?)
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Ball and stick models may look pretty, but they're too cumbersome to be used for diagramming reactions in organic chemistry. 
I weep at the sight of flaming acetic anhydride.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
| Quote: |
I don't really understand why do it need a Chlorine?
|
Well, i know that for elimination of an H to get an alkene or alkine(double elimination) you need a halogenalkane, preferably with bromine, because it
is a good leaving group(this is because it has the lowest electronegativity of the halogens, so the difference in electronegativit with the low
electronegative carbon, which holds the bond, is smaller.
-But to tell you no shit i had to look it up again ... the main reason for the halogen is that it attracts electron density from the surrounding
carbon(s), so the C-H bondings in the "neighbourhood" are considerably weakened and a strong base like OH-(or much better, an alkoxide with
steric hindrance, like sodiumphenolate, for instance) is then able to abstract one of these hydrogens while the halogen leaves simultaneously(E2
meachanism).
This in turn generates a double bond.
BTW, i forgot to mention that the last step in my reaction scheme would also generate the SN2-products, phenols, as byproduct.
My hope is that in this reaction the steric hindrance of the hexane-ring prevents the backside-attack, which is needed for SN2, so mainly the E2
product benzene is generated, so one could use the cheap base OH-.
A little bit weird, while i have an expensive grignard-reagent in the first step, i have to admit...
Also, i remind, strong bases like alkoxides with good steric hindrance could also be made by reacting NaOH with alcohols as these are protic solvents
and this would be far cheaper than reacting sodium metal with ethanol, for instance.
|
|
|