| Pages:
1
2 |
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Diiodoacetylene
Preperation of Diiodoacetylene.
WARNING: Diiodoacetylene is extremely toxic (ref. 2) and a shock, heat and friction sensitive explosive (ref. 2,3).
This preperation is a slightly modified version of that of Dehn (ref. 1). 3g of potassium iodide was dissolved into 40ml of distilled water in a
100ml measuring cylinder. A steady stream of acetylene produced by calcium carbide in water was bubbled through the potassium iodide solution. A 12.5%
solution of sodium hypochlorite was slowly dripped into the bubbling potassium iodide solution where by the solution turned reddish amber, then
turning pale yellow. The slow addition of hypochlorite was continued until a floculent white precipitate of diiodoacetylene fills the measuring
cylinder and the hypochlorite addition no longer turns the solution yellow (figure 1).
The precipitate was filtered, flushed with cold water and dried in a cardboard box since diiodoacetylene is light sensitive. Its also volatile and
will be lost through sublimation if left uncovered.
The slow addition of the hypochlorite is used to produce the unstable hypoiodite, which reacts with the acetylene to produce diiodoacetylene. The
equations for the reactions are as follows.
CaC<sub>2</sub> + 2 H<sub>2</sub>O --> Ca(OH)<sub>2</sub> + C<sub>2</sub>H<sub>2</sub>
KI + NaOCl --> KCl + NaOI
2 NaOI + C<sub>2</sub>H<sub>2</sub> --> 2 NaOH + C<sub>2</sub>I<sub>2</sub>
The yield of diiodoacetylene melting at 81°C from this procedure is about 88%. Losses are due to the instability of the hypoiodite which decomposes
through the following equations:
3 NaOI --> 2 NaI + NaIO<sub>3</sub>
NaOI + 3 NaOCl + NaOH + H<sub>2</sub>O --> 3NaCl + H<sub>3</sub>Na<sub>2</sub>IO<sub>6</sub>
<center><img src="http://www.sciencemadness.org/scipics/axt/diiodoacetylene-reaction.jpg">
Figure 1: Colour change, and precipitation of diiodoacetylene.</center>
References:
1) Dehn, W. (1911) JACS vol 33. 1598-1601
2) Fedoroff, B. et al. "Encyclopedia of Explosives and Related Items" vol. 7 pg. H6 & vol. 5 pg. D1298.
3) Urben, P. (1999) "Bretherick's Handbook of Reaqctive Chemical Hazards 6th edition" vol. 1, 0985.
[Edited on 7-12-2005 by Axt]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very interesting! I suppose you haven't yet tested the sensitivity, etc?
Also, you must be aware of dichloroacetylene? This can be made by trichloroethylene and NaOH IIRC. It spontaneously combusts at air. I think Polverone
discovered it by accident.
Anyway - do you know for a fact that NaOI is needed? Other than that it disproportionates quickly, would it be possible to simply use an alcoholic I2
solution, or I2 in KI, with no NaOCl?
Great work. As it's a solid, I could imagine that diiodoacetylene is a great syntheon.... were you thinking of making the never isolated dinitro
acetylene with it? 
There are of course many other things that could be done with it.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
No, I havn't tested sensitivity. (yet)
The other documented procedures for diiodoacetylene are not friendly at all, requiring liquid amonia etc. You cant use I2 as it will add to the triple
bond, giving diiodethylene.
Actually I was going to try form diiodoxyacetylene, iodoxyalkenes are known but I've never seen mention of iodoxyalkynes. But it would have to be done
by direct oxidation, as chlorination will destroy the triple bond. Not real sure how to do it. Carries a bit more ballast but it would have 0% OB like
dinitroacetylene which would already be made if it were that simple!
Tetramerisation to octaiodoxycubane  reduction with HCl to octaiodocubane,
additon of AgNO2 to octanitrocubane reduction with HCl to octaiodocubane,
additon of AgNO2 to octanitrocubane 
Im not sure what properties of substituted acetylenes are needed for the tetramerisation, but it is at least known.
[Edited on 5-12-2005 by Axt]
|
|
|
hinz
Hazard to Others
  
Posts: 200
Registered: 29-10-2004
Member Is Offline
Mood: No Mood
|
|
Very interesting.
When I read the topic I remembered that diidoacetylene was mentioned in the M.Satori's war gasses,
I loked for it there and found it at page 52. And there is not only the standart preparation method.
There is written that Bayer prepared it together with tetraiodoethylene by the action of I2 on CaC2.
CaC2 + 2I2 ==> CaI2 + I-CΞC-I
I hope that the reaction will produce more diidoacetylene than tetraiodoethylene (is on the I-CΞC-I side). Maybe someone trys it, because I've no
CaC2
BTW Is it true that you can't reply on a post by clicking on post reply button if you aren't logged in?
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Never thought to look in war gasses. It states that you can produce diiodoacetylene via C2H2 + KI + I2, if done in basic aqueous solution, with the
hydroxide producing the intermediate hypoiodite from I2. More interesting is the structure it gives, a carbene :C=CI2. Maybe a tautomer? Everwhere
else gives the basic acetylene structure I-CΞC-I.
Seems the reaction is very dependant on the solvent used. I2 in ethanol precipitates diiodoethylene. diiodoacetylene in petroleunm ether + I2
precipitates tetraiodoethylene. Diiodoacetylene in chloroform + Cl2 produces hexachloroethane.
[Edited on 5-12-2005 by Axt]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Once I read about a supposed non-hazardous preparation of dichloroacetylene, I googled and found that it is the following article:
Gefahrlose Darstellung von Dichloracetylen als Vorlesungsversuch
L. Metz
Journal für Praktische Chemie
Volume 135, Issue 3-5, 1932. Pages 142-144
Then I had a look in the "Free online journals" thread by chemoleo, and hooray, the entire "Journal für praktische Chemie" is available online for
free!
Here you can access the mentioned article:
http://visualiseur.bnf.fr/Visualiseur?Destination=Gallica&am...
It's the first time I found an article that I have been wanting for a long time, I feel proud! 
Dichloroacetylene is formed in 65% yield by dropping trichloroethylene on anhydrous KOH which is heated to 180°C in a glass tube, so effectively from
trichloroethylene vapors and KOH at this temperature. The product occasionally ignites during preparation, it is also important to keep the
temperature at exactly 180°C.
It is also possible to produce it from hypochlorite solution and acetylene, but with very tight control of reaction conditions and with slow reaction
and low yield.
Dibromoacetylene is formed much easier and faster from hypobromite and acetylene, making this a suitable method of preparation.
The actual demonstration makes use of a solution of 1,1,2,2-tetrachloroethane (30ml) in xylene (30ml) which is warmed with anhydrous KOH (16g) under
nitrogen atmosphere. Dichloroacetylene is formed and bubbled through water, the bubbles spontaneously combust as soon as they hit air. A lot of
phosgene is formed during this combustion, so a fume hood is crucial.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I have made a PDF, comments welcome (this should be considered another test):
http://www.sciencemadness.org/scipics/diiodoacetylene.pdf
PGP Key and corresponding e-mail address
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Looks nice, maybe the logo should have a higher resolution though, on my end it's fairly pixilated but it's the focus of the first page.
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I left a "2" out of the first equation. H2O should be 2H2O.
Still reckon the name needs a new line & smaller. Shouldn't the "figure 1: blah blah blah.." be below the image? looks a bit odd above it.
Umm.. how much info in the journal references do you want? do you want its title? In the JACS article it was "The Action of Diiodoacetylene on Organic
Bases" (diiodoacetylene forms complexes like iodoform, http://www.sciencemadness.org/talk/viewthread.php?tid=4578).
My original suggestions were based on the assumption that all the articles were to be in one pdf with header on all pages, thats why I thought the
title/header looked too big. Though now it looks fine individually. It doesn't look pixelated in mine (1024x786, adode acrobat 6.0 professional as
available on ftp) until zoomed 200%.
[Edited on 6-12-2005 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I stuck a movie of diiodoacetylene deflag up. You can see why it didn't earn a spot in the energetics section.
<center><a href="http://ww1.webtop100.net/~62552/xmovies.webtop100.net/banners/xmovies.html"><img
src="http://www.sciencemadness.org/scipics/axt/diiodoacetylene.jpg"></a></center>
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
The diiodoacetylene PDF (and all the others) have been updated. The logo is now in vector format, so it will zoom arbitrarily large without becoming
pixelated, though older versions of Acrobat (or those without image antialiasing turned on) will show it with rough outlines on screen.
PGP Key and corresponding e-mail address
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
This is probably a simple question, but what is the reaction mechanism for forming C2I2 from NaOI + C2H2?
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
This article has now been added to the collection. The complete collection can be found here: http://www.sciencemadness.org/member_publications/index.html
Oh, and I forgot to say earlier: the captions above the figures are the standard style of LaTeX, which I am using to assemble the articles.
PGP Key and corresponding e-mail address
|
|
|
acetate
Hazard to Others
  
Posts: 116
Registered: 27-8-2005
Location: Europe
Member Is Offline
Mood: LUMO
|
|
toxic
What is a reason that extremely toxicity of C2I2 ?(dont have references)
[Edited on 28-6-2006 by acetate]
//--- ---//
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by acetate
What is a reason that extremely toxicity of C2I2 ?(dont have references) |
Quote from <a href="http://www.chimicando.it/e-book/%5Bebook%5Dwar%20chemistry.pdf">The War Gases</a>:
"It has powerfully toxic properties, the vapour strongly irritating the mucous membranes and particularly the eyes (Nef) . Iodine separates as it acts
on the organism, though it partly penetrates the tissues without decomposition."
Doesnt tell much, though I guess there could be some analogy between its toxicity with that of the nasty iodoalkanes, check their msds.
[Edited on 5-7-2006 by Axt]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Diiodoacetylene also forms from impure KI (KI mixed with KIO3) using the same method. But even better due to the much higher solubility of NaI, is
from iodine tincture which has NaOH added until it becomes colorless, and then the solution boiled to boil off the alcohol. A small amount of the
compound made from the tincture explodes mildly under a hammer blow leaving behind yellow-blackish residue.
Diiodoacetylene is an extremely volatile and highly penetrable substance, over a day period I lost the entire gram amount despite it being in a small
cardboard box, wrapped in two separate plastic layers, then taped shut all around, and then put in a zip-lock bag in the dark and cool area in a
cement barricade. It also has a very strong smell. It is best to only use glass (beakers, funnels, etc) in its preparation and isolation, as it
contaminates plastics with its smell for a long time, unless maybe these are placed under the sun.
More about its physiological and pharmacological properties. Here were done several experiments about its toxicity and ability to stop decay: Archiv
fuer experimentelle Pathologie und Pharmakologie V. 41 (1898), pgs. 113-142. Quick notes on this from Centralblatt für die medicinischen
Wissenschaften, 37 (1899), pg. 256 and Schmidt's Jahrbücher der In- und ausländischen gesammten Medicin 263 (1899), p. 231:
When injected over a period of two days subcutaneously, the deadly dose in rabbits was found to be 0.2-0.3 g at a weight of 1500-2400 g. That would be
133 mg/kg to 125 mg/kg bodyweight. In the stomach it caused local irritation. By subcutaneous injection oedemias result in areas distant from the
injection site and it does not hinder the formation of abscesses at the injection site. It apparently affects the respiration organs adversely,
probably due to the acetylene. A part of diiodoacetylene in the body decomposes, splitting off iodine, an other part goes through the body
undecomposed. Per iodine content, diiodoacetylene gives lethal doses where iodoform has no significant action. Diiodoacetylene has highly powerful
anti-putrefaction properties, much higher than iodoform and several other tested iodine compounds, only cyanogen iodide comes close. Though they also
say it is not practical to employ due to its toxicity and penetrating odor. I’ve also felt some of its strong disinfecting properties. As I was
coming down with a cold and runny nose I took a few gentle whiffs of the vapor from the mg amounts and it almost entirely cleared up my nose within
minutes.
For comparison: all for the rabbit: Ethylenediamine subcutaneous: 500 mg/kg LDLo (lowest lethal dose). Formaldehyde (subcut.): 240 mg/kg LDLo. NH4Cl:
LDLo subcut., 200 mg/kg. H3BO4: subcut. LDLo: 150 mg/kg. Lewisite (blister agent) subcut.: LD50: 2 mg/kg. Subcut. strychnine LDLo: 700 mcg/kg.
[Edited on 13-5-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
A much more energetic compound would be diiododiacetylene:
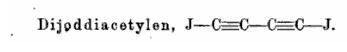
Which is mentioned in: Adolf von Baeyer's gesammelte Werke (1905), p. 698. They say It is prepared from silver diacetylide under water, which is
treated with a solution of iodine in KI, this quickly absorbs the iodine and one obtains a weakly yellow precipitate. To extract the formed compound,
the precipitate is filtered and extracted with ether. Upon evaporation of the ether, left behind is a colorless, beautifully crystallizing substance
of a melting point of 101 deg., which odor reminds one of iodoform. Analysis of C4I2 showed: I: 84.1 (found: 84.1); C: 15.8 (found: 15.9). That this
is actually an iodine derivative of diacetylene can be shown due to it yielding this hydrocarbon; if the substance is treated with Cu2Cl2 solution,
the iodine is replaced by copper, and one obtains upon warming with hydrochloric acid or KCN solution diacetylene with all of its properties.
Heating this compound in a tube causes it to explode with extreme violence giving a very unusual lightning-like red flash. Upon storage in light, it
changes to a brown mass, which seems to be soluble in no solvent. This polymer explodes when heated with a violent bang without creating fire, and
under the formation of iodine vapors and leaving behind small amounts of voluminous coal.
[Edited on 14-5-2008 by Schockwave]
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Merck confused me quite much while I came across "acetylene dichloride".
I thought it would have been the same pyrophoric gas mentioned here, but it wasn't
Acetylene dichloride
1,2-Dichloroethylene
1,2-Dichloroethene
1,2-Dichloraethen
CHEBI:18882
CAS: 540-59-0
Molecular Weight: 96.94328 [g/mol]
Molecular Formula: C2H2Cl2
while dichloroacetylene is:
1,2-dichloroacetylene
Molecular Formula: C2Cl2
Molecular Weight: 94.927 g/mol
Cas Number: 7572-29-4
1,2-dichloro-ethyne
1,2-dichloroethyne
here are a couple articles from J. Org. Chem. about dichloroacetylene
Safe and convenient synthesis of dichloroacetylene
J. Siegel, Richard Arvin Jones, L. Kurlansik
J. Org. Chem.; 1970; 35(9); 3199-3199.
Practical Synthesis of Dichloroacetylene
Jean-Noel Denis, Albert Moyano, and Andrew E. Greene
The Journal of Organic Chemistry, 52, p. 3461, 1987
[Edited on by kazaa81]
Attachment: Safe and convenient synthesis of dichloroacetylene.pdf (153kB)
This file has been downloaded 1989 times
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
couldn't attach two files in the same post
Attachment: Practical Synthesis of Dichloroacetylene.pdf (326kB)
This file has been downloaded 1773 times
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Diiodoacetylene is got to be a useful synthetic reagent.
I was wondering though, if the analogous methyliodoacetylene (or iodo-methylacetylene... CH3.C...C.I) could be prepared from methylacetylene, a
certain amount of which exists in MAPP gas.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The preparation of iodoallylene CH3.C:.C.I is described by Liebermann in J. pr. Ch. 98, 45 (Lieb. Ann. 135 [1865], 266) propyne isn't used there, the
silver salt is. A non-specific procedure, he already notes shaking some silverpropyne with aq. solution of I2 in KI, reacts and discolors
precipitating AgI and giving a terrible stench.
For the preparation, a retort is connected to a cooler, moist silverpropyne is added and through a tube a solution of iodine in KI is added until
discoloration occurs. Then upon heating, before the water vapors, an unpleasant-smelling oil goes over (comes over as a yellow and sparse oil droplets
as an emulsion) when the contents of the flask were brought to boil.
If an excess of I2 is used, upon heating there was eventually discoloration, but in the condensing tube white crystals (this is C3H3I3) formed in
small amount. The oil which was obtained in small amounts was completely pure after a small excess of iodine was removed through dilute NaOH solution.
The iodine analysis was carried out with burned marble. 0.6504g of compound gave 0.9176g AgI, corresponding to 76.26% (calculated 76.50% from C3H3I),
etc.
The pure oil has a sp. gr. of 1.7 and boils at 98 deg. It has the stenching odor and irritating properties of Berend's acetylene iodide
(diiodoacetylene). It has little solubility in water and alcohol, but is easily soluble in ether and glacial acetic acid. Water absorbs "its
characteristic odor", where it will decolorize small amounts of I2 forming a small, colorless glancing crystals. Glacial acetic acid absorbs it
easily, but it precipitates unchanged if water is added. The oil is volatile and will evaporate alongside alcohol or ether, for example. It doesn't
burn (Liebermann claims it's not flammable), solubilizes I2 with a red coloring which soons vanishes. Br2 reacts under hissing and ignition. It is not
changed by: aq. KOH (even on heating), sodium and sodium alcoholate. With zinc and hydrochloric acid or zinc amalgam it develops allylene (propyne).
In a lengthy paper, J.V.Nef in Lieb. Ann. 308 [1899] 264/328, 309 states Liebermann's iodoallylene had very different physical properties, his was
impure, a mixture of Ag2C2 and AgC3H3. Nef made it as follows:
a solution of 17.2g iodine and 11.6 g KI in 23g H2O is added slowly under shaking to 10 g C3H3Ag which is suspended in 10g H2O. Then immediately a
part of the water is distilled off, as long as oily drops come over. The oil is separated from the water, washed with dilute NaOH aq., and dried with
CaCl2. 31.1g C3H3Ag yield 28g of oil, which will boil at 109-110 deg. without the smallest decomposition. Nef characterizes it so: colorless, strongly
sweet smelling, non-poisonous oil with a spec. gr. of 2.08 at 22 deg. where according to Liebermann, Bp. was 97 deg. and sp. gr. 1.70. And where
Liebermann states it has the stenching odor and irritant properties of diiodoacetylene, Nef says this is enough to confirm his was impure. Nef
confirms triiodopropylene forming from I2 and the compound.
Though Nef and Liebermann are both wrong in claiming C3H3Ag isn't explosive, it is, but it is much safer and weaker than Ag2C2. Also significantly
less sensitive to shock. And that would be a short little history lesson about the compound. A little impurity goes a long way sometimes.
[Edited on 22-4-2009 by Formatik]
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
Would the reaction of diiodoacetylene with lithium nitrite in an ethyl ether solvent make dinitro-acetylene?
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | | Also, you must be aware of dichloroacetylene? This can be made by trichloroethylene and NaOH IIRC. It spontaneously combusts at air. I think Polverone
discovered it by accident. |
That was me, actually - seven years ago (wow, been a long time), exhibiting a laughable understanding of organic chemistry, I postulated that stirring
trichloroethylene with NaOH would yield acetaldehyde and instead found myself with a dense oil. I disposed of it immediately after finding out just
what unstable of all unholy compounds I had made: it explodes on contact with sunlight!
Interestingly, it's supposedly possible to prepare HCCCl by substituting the hydroxide with potassium carbonate. Disclaimer: old references, so who
knows if it's true. Any German speakers up for some translation duty?
Chemische Berichte, 1908, vol. 41, p. 316
Chemische Berichte, 1909, vol. 42, p. 4234
| Quote: | | Would the reaction of diiodoacetylene with lithium nitrite in an ethyl ether solvent make dinitro-acetylene? |
Dinitroacetylene sounds scary, the nitrite isomer, terrifying. The latter, which surely would be produced in some quantity, would probably
spontaneously rearrange to form... an explosion. Stick to more benign substitutions. 
I weep at the sight of flaming acetic anhydride.
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
"There will also be a few nitrites stuck on in place of the nitro group, such an arrangement is probably unstable and likely to decompose into:
ONOCCNO2 --> O=C=C(NO2)(NO)
which may be useful,
This might react with NH2OH, condensing into:
O=N--CH=C(NO2)(NO)
which then could possibly be selectively reduced with bisulfite to: HONH--CH2--CH2--NHOH
This reduction is possible through tautomers; as soon as the nitroso is reduced to a hydroxylamine group, a tautomer will form in which the nitroso
group reforms, while a hydrogen is added to a carbon, formaldoxime has a tautomer of nitrosomethane, for example."
DinitroAcetylene would also be of interest to
"PrimoPyro" in his attempt to make hexanitrobenzene.
http://www.sciencemadness.org/talk/viewthread.php?tid=259
DinitroAcetylene would probably be a very energetic compound, perhaps vaporizing rather than pulverizing whatever object happens to be in close
proximity, the explosive version of an oxy-acetylene torch.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
That's about as wishful of thinking as I've seen on here.
Forget any notion of this being utilizable for anything other than self mutilation. Electron density would migrate on formation and the energy
released by such would be so great that it's hard to see anything potentially useful existing for long enough to interact with another molecule.
Please, no one ever mix a dihaloacetylene with a nitrite - it'll just explode.
[Edited on 18-9-2010 by madscientist]
I weep at the sight of flaming acetic anhydride.
|
|
|
| Pages:
1
2 |