Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Ranque-Hilsch vortex tubes.
I discovered the Ranque-Hilsch vortex tubes last tuesday afternoon and it has blown my mind.
For those 99,99% who have never heard of it: with some pipes, fittings and compressed air you can obtain low temperatures! Check:
http://www.visi.com/~darus/hilsch/
The full article is in a book mentioned here. Wonderfull stuff BTW, thanks Poverone.
Another link:
http://www.exair.com/vortextube/vt_page.htm
I have downloaded a few papers on the subject and made a couple of prototypes. I even thought of using my vacuum pump to drive one, someone did that
and the paper is attached here (thanks andresderis).
Anyone would like to exchange ideas?
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
So, let me get this straight, you somehow put a demon inside of the tube and it separates the hot from the cold thereby making the hot hotter and the
cold colder? 
-70C is incredible, although is specifies this would take a well tuned apparatus -10 is pretty good too. Very very interesting setup, looking
foreward to seeing someone do something with one and give some positive information.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
I looked at these several years ago and thought maybe one could be used in the expansion stage of an air liquifier, to get that little bit of extra
cooling to reach liquid nitrogen temps.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
German scientists were playing with these things back in WWII, but ran into problems related to reaching very low temperatures. If there is little
differential then they have no efficiency, similar to an air conditioner not cooling well when the heat outside is so high the high side condenser
coil cannot radiate very well. Years ago I played with one I built and ran with an air compressor. If there ever was a fun mad science project this
one should be high up on the scale.
Quote from a link below: "The vortex tube was invented quite by accident in 1928. George Ranque, a French physics student, was experimenting with a
vortex-type pump he had developed when he noticed warm air exhausting from one end, and cold air from the other. Ranque soon forgot about his pump and
started a small firm to exploit the commercial potential for this strange device that produced hot and cold air with no moving parts. However, it soon
failed and the vortex tube slipped into obscurity until 1945 when Rudolph Hilsch, a German physicist, published a widely read scientific paper on the
device."
Following are some links:
http://www.exair.com/vortextube/vt_frmain.htm
http://jnaudin.free.fr/html/vtxtech.htm
http://ucalgary.ca/~kmuldrew/cryo_course/cryo_chap14_1.html
http://groups.yahoo.com/group/mad_scientist/links/Air_contro...
http://www.oberlin.edu/physics/catalog/demonstrations/thermo...
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Yes, it works.
I made two prototypes and obtained a 8ºC drop of temperature rather easily. I measured the temperature of the compressed air coming in and the cold
air coming out. It’s really hard to avoid a temperature drop, but to obtain a maximum requires some tuning.
The large prototype was made of 15mm copper tubing (12mm internal diameter) for the hot part and a scrap aluminum tube for the cold side. It uses a
conic valve made by gluing a cone to a piece of wood (see picture). The smaller one is made of 10mm aluminum tube (8mm internal) and used, as a valve,
a cone held in place by... my middle finger.
Both had nozzle “chambers” made by machining acrylic in a drill press. Both used two nozzles.
I could not obtain a larger temperature drop, probably because my pump is a simple diaphragm pump (see pic.). I don’t have a air pressure manometer,
so I can only guess the input pressure was about 2 atm. I tried cooling the hot tube with water, but got inconclusive results.
I tried a few different orifice and nozzle diameters, but any difference was shadowed by the instability of the setup.
The thermometers used were LM35 probes connected to digital multimeters as described
here.

edit: Bromic, the little demons are kept in the brown flask seen in the top picture on the left. You order them from aldrich-vodoo section.
[Edited on 5-12-2005 by Tacho]
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
I've been inspired by this thing for quite some long time since you've did this thread.
Anyway, today I've googled for the theory behind this vortex tube but I couldn't find any real explanation. I was lazy to search too much so I've
formulated an own "explanation".. Lets see if people will agree on it.. or maby I've forgot something very basic and just talking bull..
To begin with, all systems want to reach an equilibrium, be it heat transfer from hot to cold, collision distribution, or whatever. Same rules is
going on in the vortex tube.
So, in this vortex tube we have two systems composed of moving air.
Lets say system A is the outer vortex, and system B is the inner vortex:
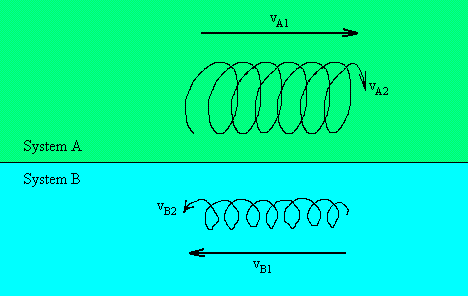
The velocities can be separated into total flow velocities: vA1 and vB1, and vortex velocities: vA2 and
vB2. These will be used later to describe kinetic energy (KE) and potential energy (PE).
Basically, we want to estimate KE and PE because these are the values that want to lie in equilibrium:
(KE + PE)sysA <==> (KE + PE)sysB
Knowing basic physics we can put together some equations that roughly describe KE and PE of both systems (correct me if I'm wrong):
System A:
KE = mvA12/2
PE = kT + mvA22/2
System B:
KE = mvB12/2
PE = kT + mvB22/2
where: m = mass of air, v = velocity (see pic above), k = some temperature constant, T = temperature of air.
where: k = some constant, T = temperature of air (in vortex), s = distance traveled by air, r = radius (of vortex)
Additional conditions, that are general for such things are that:
vA2 = vB2
vA1 < vB1
This, together with equations mentioned earlier gives:
KEsysA < KEsysB
PEsysA = PEsysB
Now to better "feel" the obtained relationships above, I have pulled some exact numbers of KE and PE out of my ass (just to illustrate the point):
Beginning (no equilibrium):
System A : KE=10; PE=100
System B: KE=100; PE=100
This means two systems are not in equilibrium, one having higher kinetic energy:
(KE + PE)sysA =/= (KE + PE)sysB
The only variables that can equilibrate between these two systems are velocity and heat (mass of air and vortex radius are constant). I guess that
velocity is not equilibrated much due to low friction (not sure). But the main variable to change in both systems is the temperature.
When equilibrating the above figures they may look something like this, here, equilibrium is fully attained:
System A : KE=11; PE=144
System B: KE=99; PE=56
This would satisfy:
(KE + PE)sysA = (KE + PE)sysB
Note, again, the exact numbers are just to illustrate the point.
The PE (temperature) has been changed alot because heat is transferred easily and KE (velocity) is altered just a little due to low friction. Sounds
fair?
This way I think that one can get same effect by injecting a high velocity air stream into low velocity air stream (with opposite direction). The only
problem would be is a shorter contact time between the two systems, which will result in not enough time to fully equilibrate..
[Edited on 23-7-2006 by frogfot]
EDIT: Stupid sub/sup script!!! They doesn't work... 
[Edited on 23-7-2006 by frogfot]
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
They are terrible loud. Actually the efficiency is directly related to the noise what suggests that there is a sonic component, resonance, in the
separation and no devil.
/ORG
[Edited on 23-7-2006 by Organikum]
|
|
|
Mr_Benito_Mussolini
Harmless

Posts: 47
Registered: 19-7-2006
Member Is Offline
Mood: No Mood
|
|
Scientific American when it was still good:
About a Remarkably Simple Device to Attain Low Temperatures and Various Other Matter
---------------------
by C. L. Stong
November, 1958
---------------------
SHORTLY AFTER THE END OF World War II word came to the U. S. that the Germans had developed a remarkably simple device with which one could reach
temperatures as low as the freezing point of mercury. The device, which was said to consist only of an air compressor and three pipes, immediately
attracted the interest of amateurs who had dreamed of performing experiments requiring moderately low temperatures. The details of construction were
not available, but it was reported that the device had in effect realized "Maxwell's demon," a fanciful means of separating heat from cold without
work.
Among those intrigued by the demon's alleged capture was George O. Smith of Highlands, N.J. Smith writes: The 19th century British physicist James
Clerk Maxwell made many deep contributions to physics, and among the most significant was his law of random distribution. Considering the case of a
closed box containing a gas, Maxwell started off by saying that the temperature of the gas was due to the motion of the individual gas molecules
within the box. But since the box was standing still, it stood to reason that the summation of the velocity and direction of the individual gas
molecules must come to zero. In essence Maxwell's law of random distribution says that for every gas molecule headed east at 20 miles per hour, there
must be another headed west at the same speed. Furthermore, if the heat of the gas indicates that the average velocity of the molecules is 20 miles
per hour, the number of molecules moving slower than this speed must be equaled by the number of molecules moving faster.
After a serious analysis of the consequences of his law, Maxwell permitted himself a touch of humor. He suggested that there was a statistical
probability that, at some time in the future, all the molecules in a box of gas or a glass of hot water might be moving in the same direction. This
would cause the water to rise out of the glass. Next Maxwell suggested that a system of drawing both hot and cold water out of a single pipe might be
devised if we could capture a small demon and train him to open and close a tiny valve. The demon would open the valve only when a fast molecule
approached it, and close the valve against slow molecules. The water coming out of the valve would thus be hot. To produce a stream of cold water the
demon would open the valve only for slow molecules.
Maxwell's demon would circumvent the law of thermodynamics which says in essence: 'You can't get something for nothing. 'That is to say, one cannot
separate cold water from hot without doing work. Thus when physicists heard that the Germans had developed a device which could achieve low
temperatures by utilizing Maxwell's demon, they were intrigued, though obviously skeptical. One physicist, Robert M. Milton, investigated the matter
at first hand for the U. S. Navy.
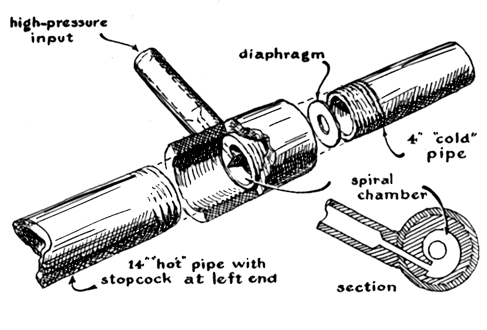
Milton discovered that the device was most ingenious, though not quite as miraculous as had been rumored. It consists of a T-shaped assembly of pipe
joined by a novel fitting, as depicted in the accompanying illustration [left]. When compressed air is admitted to the 'leg' of the T, hot air comes
out of one arm of the T and cold air out of the other arm! Obviously, however, work must be done to compress the air.
"The origin of the device is obscure. The principle is said to have been discovered by a Frenchman who left some early experimental models in the path
of the German Army when France was occupied. These were turned over to a German physicist named Rudolf Hilsch, who was working on low temperature
refrigerating devices for the German war effort. Hilsch made some improvements on the Frenchman's design, but found that it was no more efficient than
conventional methods of refrigeration in achieving fairly low temperatures. Subsequently the device became known as the Hilsch tube.
The Hilsch tube in the illustration is constructed as follows. The horizontal arm of the T-shaped fitting contains a specially machined piece, the
outside of which fits inside the arm. The inside of the piece, however, has a cross section which is spiral with respect to the outside. In the 'step'
of the spiral is a small opening which is connected to the leg of the T. Thus air admitted to the leg comes out of the opening and spins around the
one-turn spiral. The 'hot' pipe is about 14 inches long and has an inside diameter of half an inch. The far end of this pipe is fitted with a stopcock
which can be used to control the pressure in the system. The 'cold' pipe is about four inches long and also has an inside diameter of half an inch.
The end of the pipe which butts up against the spiral piece is fitted with a washer, the central hole of which is about a quarter of an inch in
diameter. Washers with larger or smaller holes can also be inserted to adjust the system.
"Three factors determine the performance of the Hilsch tube: the setting of the stopcock, the pressure at which air is admitted to the nozzle, and the
size of the hole in the washer. For each value of air pressure and washer opening there is a setting of the stopcock which results in a maximum
difference in the temperature of the hot and cold pipes. When the device is properly adjusted, the hot pipe will deliver air at about 100 degrees
Fahrenheit and the cold pipe air at about -70 degrees (a temperature substantially below the freezing point of mercury and approaching that of 'dry
ice'). When the tube is adjusted for maximum temperature on the hot side, air is delivered at about 350 degrees F.
Despite its impressive performance, the efficiency of the Hilsch tube leaves much to be desired. This perhaps explains why no one has mathematically
analyzed its operation. Indeed, there is still disagreement as to how it works.
According to one explanation, the compressed air shoots around the spiral and forms a high-velocity vortex of air. Molecules of air at the outside of
the vortex are slowed by friction with the wall of the spiral. Because these slow-moving molecules are subject to the rules of centrifugal force, they
tend to fall toward the center of the vortex. The fast-moving molecules just inside the outer layer of the vortex transfer some of their energy to
this layer by bombarding some of its slow-moving molecules and speeding them up. The net result of this process is the accumulation of slow-moving,
low-energy molecules in the center of the whirling mass, and of high-energy, fast-moving molecules around the outside. In the thermodynamics of gases
the terms 'high energy' and 'high velocity' mean 'high temperature.' So the vortex consists of a core of cold air surrounded by a rim of hot air.
The difference between the temperature of the core and that of the rim is increased by a secondary effect which takes advantage of the fact that the
temperature of a given quantity of gas at a given level of thermal energy is higher when the gas is confined in a small space than in a large one;
accordingly when gas is allowed to expand, its temperature drops. In the case of the Hilsch tube the action of centrifugal force compresses the hot
rim of gas into a compact mass which can escape only by flowing along the inner wall of the hot pipe in a compressed state, because its flow into the
cold tube is blocked by the rim of the washer. The amount of the compression is determined by the adjustment of the stopcock at the end of the hot
pipe. In contrast, the relatively cold inner core of the vortex, which is also considerably above atmospheric pressure, flows through the hole in the
washer and drops to still lower temperature as it expands to atmospheric pressure obtaining inside the cold pipe.
Apparently the inefficiency of the Hilsch tube as a refrigerating device has barred its commercial application. Nonetheless amateurs who would like to
have a means of attaining relatively low temperatures, and who do not have access to a supply of dry ice, may find the tube useful. It will deliver a
blast of air 20 times colder than air which has been chilled by permitting it simply to expand through a Venturi tube from a high-pressure source.
Thus the Hilsch tube could be used to quick-freeze tissues for microscopy, to chill photomultiplier tubes, or to operate diffusion cloud chambers. But
quite apart from the tube's potential application, what could be more fun than to trap Maxwell's demon and make him explain in detail how he manages
to blow hot and cold at the same time?
[Edited on 23-7-2006 by Mr_Benito_Mussolini]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I agree with Tacho that this seems like an incredible device! If I understand it correctly it is separating fast and slow molecules that are found
naturally in a gas due to the Maxwell distribution of velocities. If this device would have been known in the late 1800's it would have provided
tremendous support for Maxwell, Boltzmann, and Gibbs who believed in such an atomic view of matter. There was, however, at that time prominent
scientists who did not, such as Mach and Nernst.
I just learned this from perusing some of the articles provided by links that franklyn posted uner the "Entropy" thread. Also covered there is
Maxwell's Demon as well as other similar puzzles.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
Hilsch tube
I have actually read about the thermodynamics involved with this in an old back issue of scientific american. They had a monthly column about the
amateur scientist or some such. They also had several useful graphs detailing the changes needed in hole sizes to optimize this for either hot or
cold output. If someone has access to back issues of Sci-am it contained a lot of information that could be very useful in making/optimizing a vortex
tube.
Endo
|
|
|
Shingoshi
Harmless

Posts: 49
Registered: 20-4-2008
Location: Salem, Oregon
Member Is Offline
Mood: No Mood
|
|
It's almost 2 years later...
| Quote: | Originally posted by Organikum
They are terrible loud. Actually the efficiency is directly related to the noise what suggests that there is a sonic component, resonance, in the
separation and no devil.
/ORG
[Edited on 23-7-2006 by Organikum] |
And I came to the same conclusion as you have. Any system of this type, whether gaseous or fluid, must take into account the principles of acoustics
and harmonics to account for the performance of said system. Basically, we're dealing with an advanced form of air-horns. Whatever is required to
achieve greater volumes in sound, will directly influence the temperatures produced.
I am very grateful for this site, and the discussions that are provided here. Let's change the world!
Hopefully for the better,
Shingoshi
/edit: I think it should be mentioned, that there is no requirement here that only one type of gas be used in this system. Nor is
there any requirement that the system operate with only one gas. Furthermore, neither is there a requirement that the operating medium be a gas at
all. It is indeed quite possible to use a combination of gas and fluid as the operating medium.
[Edited on 2008.4.28 by Shingoshi]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Since you have flow you have work being done no mystery there. I wonder if you had two diameters of tubing the hot would go out the larger diameter
to conserve the momentum of the fluid?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
High pressure, reasonably incompressible fluid I'm pretty sure would cavitate quite nicely around the nozzle. My gut tells me density and viscosity
would prevent the hot stuff seperating from the cold stuff very easily.
Tim
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
An odd thought has just struck me. These separate gas into 2 streams according to the energy and/ or the momentum of the molecules. Do they separate
mixtures to any extent? Is one of the output streams richer in O2 and the other in N2?
Anyone in a position to do the experiment?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I doubt it. My proof: the AEC isn't regulating them. 
Tim
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
But you've got to admit it's an intriguing idea and why not? If density why not mass too?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Shingoshi
Harmless

Posts: 49
Registered: 20-4-2008
Location: Salem, Oregon
Member Is Offline
Mood: No Mood
|
|
Gases for the masses...
| Quote: | Originally posted by chemrox
But you've got to admit it's an intriguing idea and why not? If density why not mass too? |
That's exactly the point I was raising about using more than one type of gas. This system doesn't care what's pumped through it (as long as it's not
highly-corrosive).
So think of taking two gases, one with a heavy molecule and one with a light molecule. The heavier gas molecules will move to the perimeter of the
tube due to centrifugal forces. This will leave the majority of the lighter molecules in the center, giving up their excess energy (in heat) to the
larger molecules on the perimeter. Using such a system would only enhance the efficiency of it. The heavier molecules having greater mass, can absorb
more heat from lighter ones. This is an advantage not possible in a single gas system.
Shingoshi
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
I believe some newer more energy-efficient refrigerators using a vortex cooling system driven by an ultrasonic transducer, though I'm not entirely
sure where I saw this information.
A wee reference: http://en.wikipedia.org/wiki/Thermoacoustic_hot_air_engine
|
|
|
Shingoshi
Harmless

Posts: 49
Registered: 20-4-2008
Location: Salem, Oregon
Member Is Offline
Mood: No Mood
|
|
My deepest apologies to everyone with whom I previously communicated here. I hope that I will be back for good now.
Shingoshi
|
|
|