kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Naphthalene derived compounds......?
Hallo to all,
I'm interested on compounds made from naphthalene (C10H8), for example compounds with naphtyl radical.
For now, I only have idea to boil naphthalene in conc. (90%) H2SO4 to get a naphtalene-sulphonic acid, then neutralize it with NaOH to get the Na salt
of this acid.
Please help posting any information and idea!
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
Dunno if I posted this somewhere already.. but I've always wanted to try out this route:
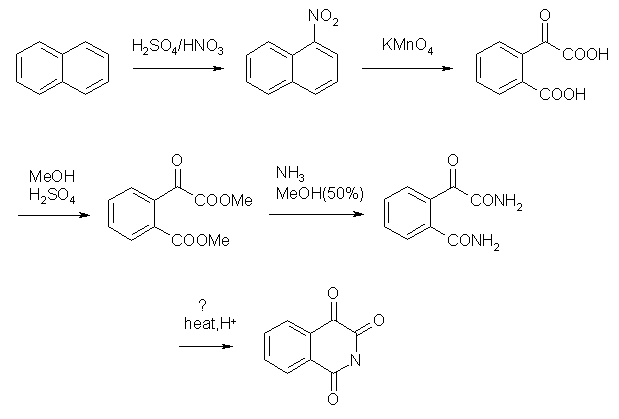
First two steps nitration and oxidation are described in litterature. The naphthalene could be oxidised by KMnO4 stright away to that dicarboxylic
product but yield is low. It becomes somewhere around 70% if you oxidise nitronaphthalene instead. Next steps are not tested I think.
Third step is esterification, done in excess of methanol with catalytic H2SO4. Procedure may be the same as for preparation of methyl benzoate.
Next, amidation, has no reason to not work. It's done in 50% MeOH saturated with NH3.
The last step is a bit doubtfull... thogh since it's intramolecular, it may go with high yield.. There should be H+ source to remove forming NH3.
Maby boiling the diamide in dilute H2SO4 will help or plain water.. though there will be problem with solubility.
The reaction steps are quite simple and not that hazardous. There may be a problem with steps 3 and 4 to see the compleation of reaction. Without TLC
you can't tell if reaction proceeded with both carboxylic groups or both ester groups..
Although there should be some easy ways to isolate the products in these reactions.. in 3rd step, simple base wash will remove both starting material
and mono ester.
In 4th step it feels that product can be separated by crystallisation. The ester is most probably an oil while amide is crystalline (just a guess with
regard to similar compounds). So hopefully, the amide will crystallise nicely on evaporation of methanol.
Knowing the mass of isolated product you can easily adjust the reaction time to get best yields.. well this includes some work..
[Edited on 6-11-2005 by frogfot]
[Edited on 6-11-2005 by frogfot]
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Thank you frogfot, but this procedure (last steps) seem too difficult to manage for me...
Any other idea about compounds from naphthalene welcomed!
Thanks all for help!
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
You can add sodium metal to napthalane in a polar aprotic solvent like THF and obtain the radical anion of napthalene
[C<sub>10</sub>H<sub>8</sub>]<sup>-</sup>:
Na + C<sub>10</sub>H<sub>8</sub> ---> Na(C<sub>10</sub>H<sub>8</sub>
I've heard that it is a blue color, not sure the the synthetic possibilites but I like blue  The solvent though is fairly important, in a non-polar medium like benzene I got no reaction between dissolved
napthalene and sodium metal. The solvent though is fairly important, in a non-polar medium like benzene I got no reaction between dissolved
napthalene and sodium metal.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Thank you, bromic!
This one seem good and easy!
Anyone know more about naphthalene sulphonic acid/salts or other things to do with naphthalene?
Thanks all for help! 
|
|
|