Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Benzophenone
A lab report
A. Introduction
This preparation follows the procedure in Brewster (ref 1). This is a small scale synthesis (20-25g) compared to those in Vogel (105g) and OrgSyn
(490-550g). The precursors are benzene and CCl4. The Lewis acid catalyst is anhydrous AlCl3. The initial product is benzophenone dichloride.
According to Wiki this is a double Friedel-Crafts alkylation (ref 2). This product is then hydrolyzed to benzophenone.
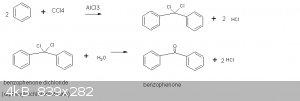
The amount of benzene used is limited so that a third phenyl group does not add to the dichlorocarbon center.
B. Reaction A 500 mL RBF equipped with a Claisen adaptor, reflux condenser, and p-e addition funnel was mounted over a magnetic
stirrer. A CaCl2 guard tube was affixed to the top of the reflux condenser to prevent moisture from reaching the reaction vessel.

reaction vessel set-up
The HCl gas exiting from the guard tube was led to an inverted funnel discharging onto the surface of 400 mL of 10% NaOH in a 600 mL beaker.

HCl absorbtion trap
25 mL of CCl4 was first placed in the reaction vessel. Then 35g of anhydrous AlCl3 was quickly added directly from its original container using a
powder funnel. The AlCl3 container was placed on a digital scale so that the amount added was obtained by weight difference. The powder funnel was
then rinsed clean by adding another 25 mL of CCl4. Good mixing of the covered AlCl3 was then attained with the magnetic stirrer. This technique
worked very well for minimizing the exposure of the anhydrous AlCl3 to atmospheric moisture.
A mixture of 30 mL each of CCl4 and benzene was then prepared. Both had been previously dried over CaCl2 overnight. This mixture was then added to
the p-e funnel. The mix was carefully added to the reaction vessel 10 mL at a time. A plastic vessel was in place in case the mix needed to be
cooled. A Bunsen burner was ready in case heat was needed to start the reaction – it was not. Upon addition of the CCl4/benzene the reaction
started right away as evidenced by temperature rise, bubbling, and the appearance of a blood red color.

initial reaction appearance
Brewster specified adding the reactants at such a rate that the reaction mix maintained a temperature of 30-40°C as detected by hand. This was
readily achieved. I did not cool the vessel but should have as the temperature did overheat somewhat toward the end of the addition. Toward the end
of the addition the mix had charred somewhat and turned a dark brown. Following the addition of the CCl4/benzene the reaction mix was heated on a
steam bath for 20 minutes to complete the reaction.

heating on the steam bath
C. AlCl3 Destruction
The cooled reaction vessel was then surrounded by an ice-bath, and 50 mL of water was added to the p-e addition funnel. This water was then added
carefully drop-by-drop to destroy the residual anhydrous AlCl3. This reaction was violent in typical fashion of active AlCl3, generating clouds of
moist HCl smoke. The CaCl2 guard tube was removed at this time lest it become plugged. The condenser effluent was then routed directly to the HCl
absorption trap. Near the end of the reaction I noticed a sizeable, mostly submerged, floating white mass having the consistency of butter. This
apparently was the benzophenone dichloride. The product mix was then sealed and left overnight.
D. Steam Distillation
The next day equipment was set up for steam distillation to remove the remaining benzene/CCl4 and to effect the hydrolysis. It was first necessary to
transfer the product mix to a larger vessel, ie, a 1000 mL RBF. The buttery solid (see picture below) had to be transferred laboriously by spatula
due to the small opening (19 mm) of the reaction vessel.

buttery white solid
The steam distillation was conducted for a full 50 minutes to effect a complete hydrolysis. All of the buttery solids disappeared,
melting/hydrolyzing during the steam distillation. About 60 mL of CCl4 containing some benzene was recovered from the 2 phase distillate.

steam distillation set-up
After cooling the pot contents were transferred to a 500 mL separatory funnel. The picture below shows the two phases.

separation of the benzophenone and aqueous layer
The lower aqueous layer was saved and later returned to the separatory funnel. It was then extracted with 33 mL of benzene. This extract was added
to the benzophenone. A full spatula of anhydrous MgSO4 was added to the benzophenone/benzene for drying. It was then sealed and left overnight.
E. Vacuum Distillation
The benzophenone/benzene mix was transferred to a 100 mL RBF. As it was transferred it was filtered to remove the hydrated MgSO4. It was subjected
to simple distillation to remove the benzene. 22 mL of hazy benzene was recovered.
The benzophenone was then subjected to vacuum distillation at P=1-2 mmHg. The product came over at 122-124°C. It was necessary to use an ebulliator
tube to prevent bumping. The picture below shows the distillation set-up. No cooling water was used as the mp of benzophenone is 48.1°C. Neither
was it necessary to heat the condenser although I did set up to blow hot air through it if necessary.

vacuum distillation set-up
F. Results
The yield was 22.1g in agreement with that expected by Brewster. This is a 72.3% yield on benzene, the limiting reactant.
G. Discussion
This is my 2nd try at making benzophenone. I used this same procedure on the first try only at ½ scale, but it failed. Since I had used homemade
CCl4 I didn’t know if this was the cause or if it was due to inactive AlCl3. I used homemade CCl4 for this 2nd run also, but my AlCl3 was fresh
material from Elemental Scientific. I now firmly believe that the earlier failure was due to bad AlCl3. This is a very pleasing result not only for
this synthesis but because it also validates my synthesis of CCl4.
If I would ever do this synthesis again I would pay more careful attention to maintaining the correct temperature range during the initial reaction.
The Vogel and OrgSyn preps specify a lower temperature range (5-10°C) for this than the Brewster procedure. But the main problem was caused by me
not keeping the reaction mix within the 30-40°C range near the end of the benzene/CCl4 addition. The result was excess tar (~ 1 mL).
H. Safety
It should be noted that the HCl absorption trap was completely overwhelmed during both the initial reaction and during the AlCl3 destruction step.
Therefore this procedure must be conducted in a good fume hood or outside.
I. References
1. Unitized Experiments in Organic Chemistry, (1960) by Brewster et al.
2. Wikipedia: http://en.wikipedia.org/wiki/Diphenyldichloromethane
Comments, questions, and suggestions are welcomed.
[Edited on 1-11-2014 by Magpie]
[Edited on 1-11-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Great synthesis, writeup and documentation. I wish the people I work with could write up an experimental anywhere near that well. The temperature
control area is where I have the most problems in many reactions, as often there is a time lag or delay in the exotherm. But you did fine to get a
72% yield, that is good for a 2 step reaction with a distillation.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks, Dr Bob. Retirement is great: I have all this time to spend in the lab and write lab reports. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Everything Bob said and also I love the copper steam bath-I want one!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you. Keep your eye pealed on eBay - that's where I bought mine.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
I concur with Dr Bob on your efforts, particularly with regard to your always good write ups. Clearly you are "old school."
And retirement is great.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you AvBaeyer. And yes I'm "old school." I don't even have a cell phone (but my wife does). I think I'm permanently stuck in the 60's.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Nice work. Now make some benzopinacol. 
http://www.orgsyn.org/Content/pdfs/procedures/cv2p0071.pdf
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Also: benzophenone oxime ---> benzanilide
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very nice work and write-up, Magpie.
What causes the reddish colouration? Neither the intermediate nor the end product are colourful.
Re. absorbing HCl with the inverted funnel method, your work shows the limitations of that method when HCl load is high, I guess. When I prepared HCl
gas with the usual NaCl/H2SO4 generator I used an anti-suckback vessel and immersed its outlet tube directly into water. The stream of HCl didn't even
form any bubbles, so immediate and complete the absorption of the HCl into the water was. It just formed one small bubble, with HCl transferring
across the gas/water interface!
[Edited on 2-11-2014 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have no idea.
Your submerged outlet device was likely more efficient than my inverted funnel. But the HCl gas generation for this synthesis was more intense than
anything I had seen before.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
If I'm not mistaken you used 0.337 mol of benzene, generating (acc. stoichiom.) 0.337 mol HCl, probably less than what I generated (I seem to recall
my objective was 0.5 mol to be captured in 500 ml water). Of course one has to take into account the rate of generation too.
It's a damn shame these FC reactions demands so much AlCl3 per mol of reagent.
[Edited on 2-11-2014 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, the HCl would come over in spurts right after I added 10 mL of benzene/CCl4. This certainly could have been evened out by adding it dropwise.
About the AlCl3: Yes, it takes a lot, then you purposely destory it by adding water. I must say that the fresh anhydrous AlCl3 that I just received
from Elemental Scientific was "dynamite."
When I rinsed off the spoon (used to add it) under the water tap there was a dramatic poof.
I know you were working on a synthesis of AlCl3. Might you give us a progress report?
[Edited on 2-11-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Great job Magpie!
Again, compliments to your report. Organized well. 'Tis a shame these things don't get published.
|
|
|
lullu
Hazard to Self
 
Posts: 51
Registered: 2-3-2012
Member Is Offline
Mood: No Mood
|
|
very nice Magpie!
May I ask how you generate your steam for destillation?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Hi lullu,

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I'd like to chime in on the well deserved applause.
I suspect the red-brown colouration of the reaction mixture arises from a combination of R+[AlCl4]- adducts and the usual organic tars.
I agree with your comments regarding AlCl3. If you're worried about its useability, you should purify before use by sublimation under reduced
pressure. I've been wondering if its possible to avoid the quench, and isolate an AlCl3-rich material by e.g. filtration. I suspect an appropriate
slurry treatment/adduct formation and subsequent purification, followed by sublimation under reduced pressure (if necessary) would give material of
adequate activity for re-use.
If AlCl3 wasn't so volatile you might have been able to distill the reaction mixture under reduced pressure to afford a (semi-)solid residue suitable
for further processing.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
What remains to be done is to check how well it ['my' AlCl3] behaves in FC reactions. I might just try the first part of your synthesis, that appeared
very fast. Still haven't got significant amounts of benzene though... Might try toluene, just to see.
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
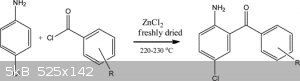
If i use only aniline will i get 2aminobenzophenone?
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
But how do you prevent acyl chloride to react with amine?
|
|
|