| Pages:
1
2
3
4 |
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Lanthanum from pool cleaner
...because diddi's account accidentally got deleted, and we need to continue research.
Carry on.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
didi needs to get a new account and PM Polverone- Then he can re-set password & get anything not permanently deleted back up.
See here:
http://www.sciencemadness.org/talk/viewthread.php?tid=37991&...
Quote: Originally posted by Polverone  | I am very sorry, but your profiles were temporarily deleted. I have reactivated your accounts, but you will need to send me a U2U through a temporary
account or email me to get your new password. My email address is gfxlist@yahoo.com
I am sorry for the inconvenience. |
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
AsocialSurvival
Harmless

Posts: 43
Registered: 8-10-2014
Member Is Offline
Mood: No Mood
|
|
If I wanted Lanthanum, I would get it from soil, rocks, or flint from lighters! Flint (not mineral with the same name) in lighters is mixture of La,
Ce, Pr, Nd. Soil contains La in miligram amounts.
[Edited on 13-10-2014 by AsocialSurvival]
|
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by AsocialSurvival  | If I wanted Lanthanum, I would get it from soil, rocks, or flint from lighters! Flint (not mineral with the same name) in lighters is mixture of La,
Ce, Pr, Nd. Soil contains La in miligram amounts.
[Edited on 13-10-2014 by AsocialSurvival] |
Or, you know, you could just buy a perfectly OTC and relatively pure source of lanthanum. But by all means, attempt to extract significant quantities
from soil in an amateur setting. Have fun!
The lighter flint is something I've attempted before, and separating the 50% Ce, 25% La, 1% other RE's and balance of Fe and Mg is no easy task.
I wondered why I couldn't find the relevant thread here for a while... Never did see if anyone responded to my question of getting a smaller (but
presumably more concentrated) amount of phosphate remover or a larger (but specified as diluted) different brand.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Texium
Administrator
       
Posts: 4675
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Quote: Originally posted by AsocialSurvival  | If I wanted Lanthanum, I would get it from soil, rocks, or flint from lighters! Flint (not mineral with the same name) in lighters is mixture of La,
Ce, Pr, Nd. Soil contains La in miligram amounts.
[Edited on 13-10-2014 by AsocialSurvival] |
Good luck purifying visible amounts of it from those sources.
Lanthanides, in case you weren't aware, are extremely hard to separate chemically. Also, the tiny amounts found in most soil would be so miniscule and
hard to isolate that you might as well try and find gold in your soil.
|
|
|
AsocialSurvival
Harmless

Posts: 43
Registered: 8-10-2014
Member Is Offline
Mood: No Mood
|
|
OK, anybody interested in these procedures, feel free to subscribe to my yt channel: http://youtube.com/user/AsocialSurvival
Also, I will never buy anything for Chemistry, I will only sell something. Chemistry's purpose, in my case is to become rich, not poor.
[Edited on 14-10-2014 by AsocialSurvival]
|
|
|
Texium
Administrator
       
Posts: 4675
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
I'm eager to see these procedures, but unfortunately it appears that your YouTube channel doesn't exist.
Anyway though, how is it that you expect to isolate lanthanum from your soil?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Hey, it worked for Alfred Nobel. But Nicolas Leblanc, not so much. Let us know how this pans out for you...
BTW, YouTube link is not working.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
AsocialSurvival
Harmless

Posts: 43
Registered: 8-10-2014
Member Is Offline
Mood: No Mood
|
|
First dissolve soil or rock in Nitric Acid (which I have in unlimited quantity). That would dissolve almost all elements present there, except Si, Ti
and few more. The rock will immediately disintegrate into insoluble powder. Then, by boiling solution of soluble elements, I could perfectly seperate
them all. It's long explanation why would only Nitric acid in small quantity would be needed, and boiling that all, but once again, video will come
one day! Fixed: http://youtube.com/user/AsocialSurvival
[Edited on 14-10-2014 by AsocialSurvival]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Oh, the YouTube channel is there. Just no content of any type.
"Unlimited (free?) nitric acid"... Perhaps means someone else is paying for nitric acid they are not getting? Did you surreptitiously tap into an
explosives factory pipeline or what?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Tdep
National Hazard
   
Posts: 520
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Surely you could just sell your unlimited nitric acid and get rich that way?
But we're getting distracted. The only method people said was viable was precipitating the fluoride and then reducing it with lithium?
This can all be done OTC in Australia then, yielding a few grams of lanthanum metal for about $50. Not cheap but an interesting project
|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
@ AsocialSurvival
You have a lot more confidence in your ability to separate compounds than I do. One of the characteristics of the REs is their similarity in chemical
and physical properties which is why, historically, they have been so hard to isolate. (Hence the challenge and interest that they present.)
I am envious of your supply of nitric acid. Wish I had the same kind of resource. I have to make mine.
One of the appeals of using OTC LaCl3 is it enables some experimentation without going through the difficult process of isolating. And indications
are that reduction of LaCl3 to La is by no means a trivial process in itself. If posters' experience with Nd is anything to go by, this is a
difficult task in a home lab situation.
Given that the original thread disappeared into the ether, let me summarise what I remember of the findings.
- Lanthanum Chloride is available OTC – sold as a swimming pool additive to precipitate phosphates that can then be filtered out. Interesting
use of the chemical.
- A likely route for refining is to convert to LaF3 and reduce with lithium. Potassium has also been suggested.
- The thermodynamics for reduction directly from LaCl3 are questionable. Blogfast reported a paper that had some success with CeCl3. No hard
data presented for La.
- LaCl3 exists as a heptahydrate and is difficult to crystallise. It is extremely hygroscopic. LaF3 is insoluble which is another reason for
suggesting this route.
- Precipitating LaF3 could conceivably be done using a fluoride salt. NaF possible but NH4F2H is suggested. This is available OTC as glass
etchant and tile roughener. In Aus, Home Hardware sells a litre at 48g/L for around $27. I found a chemical supplier who will sell for $71 plus
freight for 500g. It is available in bulk bags of 25kg each. I don't think i need that much.
- Blogfast has begun work with 2.5L of LaCl3 solution that he bought. I will let him post the details. Looked very interesting though.
- Some side discussion on the logistics of storing rare earths was also had. Standard techniques of ampouling under argon or oil seemed to be the
way to go.
- I am sure I have missed something. I am fascinated by the whole project and while my participation in practical experimentation will probably
have to wait for a few months, I am interested in the ideas offered. It seems like the original thread was of significant interest to a number of
people on this board.
Now. Let me not die again and take my posts with me.  In the interests of
keeping this topic alive does someone want to quote this post so that if another glitch happens the information will not disappear. In the interests of
keeping this topic alive does someone want to quote this post so that if another glitch happens the information will not disappear.
Blogfast, if you remember what you posted before, I would love to take a look at some of those links.
J.
|
|
|
AsocialSurvival
Harmless

Posts: 43
Registered: 8-10-2014
Member Is Offline
Mood: No Mood
|
|
Supply of nitric acid is simple, air contains nitrogen, wood gives energy.
Why are you all complicating this? So simple! Rare earth metals are easy to seperate just like any other element. I am confident in that that I can
separate them all using only Nitric acid!
What fluorides? What Lithium? That is too complicated. OK, let's see how would I separate all rare earth metals?
Just add enough acid for one to dissolve (if all others are carbonates at the beginning). For reduction use K or Na or Li or Rb or Cs or electricity
or something else. There're many ways.
Just like scientists complicate nuclear transmutation and want to force us to believe it is hard or impossible or complicated or a big deal. Not
really! Some people just want to put me down, to force me to believe in negativity, and to waste my life trying and not succeeding. It's not about
complications, not really - it's easy to do many things much more than you all think. But everybody will tell you "impossible", "complicated",
"expensive".
Here's my promise for everybody: I won't talk with you until I prove to you that many things are possible. Next time I come here, I will show you how
to separate all elements from soil or rocks using nitric acid only, and to make gold with nuclear fusion to become rich.
Bye until than!
|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Thanks for your thoughts AsocialSurvival.
I rather suspect you are missing a few critical things. It is not like we are making things complicated just for the hell of it. Things become
complicated out of necessity because they are difficult. Chemists consider energy changes that are required for chemical reactions. And that is why
energetic materials like potassium, lithium and fluorine are needed. If you can obtain nitric acid from atmospheric nitrogen and energy from a wood
fire then you really need to patent that. Reaction mechanics suggest that there are quite a few obstacles in that path.
And your promise... That sounds like a reasonable challenge you have set yourself. Good luck.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
Just like scientists complicate nuclear transmutation and want to force us to believe it is hard or impossible or complicated or a big deal. Not
really! Some people just want to put me down, to force me to believe in negativity, and to waste my life trying and not succeeding. It's not about
complications, not really - it's easy to do many things much more than you all think. But everybody will tell you "impossible", "complicated",
"expensive".
|
There is chaos under Heaven, and the situation is excellent...
You go, son.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Before I resume my actual contribution here, this little gem below merits a short answer:
Quote: Originally posted by AsocialSurvival  | What fluorides? What Lithium? That is too complicated. OK, let's see how would I separate all rare earth metals?
Just add enough acid for one to dissolve (if all others are carbonates at the beginning). For reduction use K or Na or Li or Rb or Cs or electricity
or something else. There're many ways.
Just like scientists complicate nuclear transmutation and want to force us to believe it is hard or impossible or complicated or a big deal. Not
really! Some people just want to put me down, to force me to believe in negativity, and to waste my life trying and not succeeding.
|
You are a nut and possibly need help. Possibly a troll.
But do by all means come back when you've actually got something... I won't be holding my breath though.
[Edited on 14-10-2014 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I’ll summarise my work on the lanthanum bearing (10.3 % La glycolate, acc. MSDS) pool phosphate remover called ‘Lo-Chlor Starver’ (from Lo-Chlor
Chemicals, AU) as follows.
2.5 L of this solution was neutralised with 33 % NH3 and the snow-white precipitate, presumed lanthanum hydroxide, quantitatively Buchnered and
washed. This yielded about 1 L of product.
It was dissolved gradually and quantitatively in 280 ml HCl 37 % with mild heating, followed by simmering. It dissolved completely to a clear solution
that was… slightly green! On further boiling that colour changed to yellow. I have very strong reasons to believe this colour is due to MORE than a
bit of ferric chloride, more about that later. Possibly there is Pr present.
The solution (about 1.25 L) was then gradually boiled down to about 290 g, by which time it was urine yellow. On refrigeration a mass of small, white
crystals formed (photos later) although much of the presumed lanthanum chloride heptahydrate remained in solution, as expected. The rest of the LaCl3
will now be recovered using the double sulphate method (with K2SO4), to try and separate it from the yellow contamination. If it cannot be separated
I’m inclined to believe the original phosphate remover contained also Praseodymium. If it can, that would point to ferric chloride.
As regards possible methods of preparing La (and other REE) metals, I proposed the reduction of LaF3 with Li, which appears to be a thermodynamically
favourable reaction, possibly with enough reaction heat to ensure clean separation between metal and slag. LaF3 is water insoluble, so fairly easy to
prepare.
I earlier dismissed the use of reduction of anh. RECl3 but Brauer’s ‘Handbook of Preparative Inorganic Chemistry’ proved me wrong on that. I
suggest strongly that anyone interested in contributing to this discussion go to the Library of this site, download Brauer and print off pages
1135 to 1150 from Volume 2.
There is an absolute wealth of information there that seems to have been overlooked by the ‘RE nutters in residence’ so far, including myself.
Reduction with K, with Ca, alcoholic electrolysis of RECl3 solutions with Hg cathode to REE amalgam and details on electrolysis of anh. RECl3/KCl
melts are but a few topics covered. Details on preparing anh. RECl3 is also covered. And for the very curious (Brain&Force?): preparation of some
REE(II) salts too, like EuSO<sub>4</sub>.
[Edited on 14-10-2014 by blogfast25]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Does anybody else think we have a new resident troll here on SM?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This was the LaCl3 solution at the start of the boiling process, light green:

Here after concentrating and chilling, with crystals of presumed LaCl3.7H2O on the bottom. The solution is over 50 w% LaCl3:

[Edited on 14-10-2014 by blogfast25]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I am not that new FFS 
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Would they be just above the neck?
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Maybe if he could manipulate matter with will alone he might get somewhere. Otherwise, he doesn't stand a chance.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Please let us now restrict further remarks to the science and engineering aspects of the OP's topic.
If being trolled, DO NOT FEED THE TROLL. It will go away or die.
If site rules are violated, user that little blue "report" button or PM a moderator.
And I JUST LOVE the swimming pool chemical supply section at various stores! Right up there with the ceramic glaze supply, photographic supply and
auto supply stores.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Bert  | Please let us now restrict further remarks to the science and engineering aspects of the OP's topic.
If being trolled, DO NOT FEED THE TROLL. It will go away or die.
If site rules are violated, user that little blue "report" button or PM a moderator.
And I JUST LOVE the swimming pool chemical supply section at various stores! Right up there with the ceramic glaze supply, photographic supply and
auto supply stores.
|
I wish I had the 2nd and 3rd ones 
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by blogfast25  | I’ll summarise my work on the lanthanum bearing (10.3 % La glycolate, acc. MSDS) pool phosphate remover called ‘Lo-Chlor Starver’ (from Lo-Chlor
Chemicals, AU) as follows.
2.5 L of this solution was neutralised with 33 % NH3 and the snow-white precipitate, presumed lanthanum hydroxide, quantitatively Buchnered and
washed. This yielded about 1 L of product.
It was dissolved gradually and quantitatively in 280 ml HCl 37 % with mild heating, followed by simmering. It dissolved completely to a clear solution
that was… slightly green! On further boiling that colour changed to yellow. I have very strong reasons to believe this colour is due to MORE than a
bit of ferric chloride, more about that later. Possibly there is Pr present.
The solution (about 1.25 L) was then gradually boiled down to about 290 g, by which time it was urine yellow. On refrigeration a mass of small, white
crystals formed (photos later) although much of the presumed lanthanum chloride heptahydrate remained in solution, as expected. The rest of the LaCl3
will now be recovered using the double sulphate method (with K2SO4), to try and separate it from the yellow contamination. If it cannot be separated
I’m inclined to believe the original phosphate remover contained also Praseodymium. If it can, that would point to ferric chloride.
As regards possible methods of preparing La (and other REE) metals, I proposed the reduction of LaF3 with Li, which appears to be a thermodynamically
favourable reaction, possibly with enough reaction heat to ensure clean separation between metal and slag. LaF3 is water insoluble, so fairly easy to
prepare.
I earlier dismissed the use of reduction of anh. RECl3 but Brauer’s ‘Handbook of Preparative Inorganic Chemistry’ proved me wrong on that. I
suggest strongly that anyone interested in contributing to this discussion go to the Library of this site, download Brauer and print off pages
1135 to 1150 from Volume 2.
There is an absolute wealth of information there that seems to have been overlooked by the ‘RE nutters in residence’ so far, including myself.
Reduction with K, with Ca, alcoholic electrolysis of RECl3 solutions with Hg cathode to REE amalgam and details on electrolysis of anh. RECl3/KCl
melts are but a few topics covered. Details on preparing anh. RECl3 is also covered. And for the very curious (Brain&Force?): preparation of some
REE(II) salts too, like EuSO<sub>4</sub>.
[Edited on 14-10-2014 by blogfast25] |
I'm actually downloading some references from the library for the first time. Frankly, this is embarrassing. I don't have europium (yet) but I will be
making some EuSO4 shortly after I have some.
blogfast25, potassium sulfate may not be the ideal reagent for precipitating out the ferric ions. The sulfates of very light REEs appear to be
relatively soluble, per the data I linked earlier:
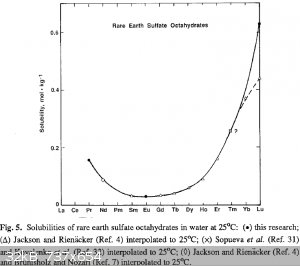
The oxalate method may be preferable, as all lanthanide oxalates are ridiculously insoluble.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
| Pages:
1
2
3
4 |