| Pages:
1
2 |
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Electrode material that won't oxidize?
I need to figure out what material I can use for electrodes, that will last at possibly close to 1600*C in air -- without burning up in the air or
becoming covered by a nonconductive layer through reaction with the oxygen and/or nitrogen under these conditions. I intend it to be in half mm thin
rectangular form.
Platinum seems to fit the conditions, but even a foil a tenth of the thickness I need costs $100 from what I found. What about tungsten? It can
withstand much higher temperature, but it burns in air... So what to use? Is there some reasonably priced alloy or something?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Why does it need to be this thick? If I were you, I'd go down to my local jewler, get a 1.5g platinum wedding band for $35, and hammer it out to
the needed size. Remember that Pt is ductile, so this isn't so hard. If you don’t feel like hammering, you might want to consider a 1 gram Pt
bar, they’re sold on Ebay for ~$35.
Else, I'd try plating a piece of something (tungsten wire is cheap) with Pt, that might work.
If you need real corrosion resistance (on the order of HF and aqua regia), get a piece of PtIr alloy. Unfortunately, this isn’t so cheap because
both of these metals are precious metals.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
I just need something to withstand that temperature in AIR, without getting an oxidized or nitride layer thick enough to prevent electrical flow into
the discharge (nor, on the other hand, eroding away). I know platinum doesn't react with oxygen under normal temperature, but I couldn't
find data for such high temperatures.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
What kind of conductance are you aiming at? Because anorganic nitrides are conductors, albeit bad ones. Otherwise check tantalum.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Quince
electrodes, that will last at possibly close to 1600*C in air I intend it to be in half mm thin rectangular form.
|
i am currious what you would be doing that only gets to 1600 C, runs in air, and uses electrodes? (and AC or DC?)
Copper would work, if it was able to get rid of heat quickly enough. (by either calculating volume of the Cu, the thermal conductivity and time as the
variable, or fixed time with volume as the variable).
Ta, Pt, Ir, Pd, Moly, Tungsten, Zr (I think) to name a few. There are Moly/Ni steels that can withstand oxidation at those temps. Getting most of
these metals into small flat sections might be a problem though. Ta and Pt are easy to roll, but Ir, Pd, Mo, W are all very brittle metals, Zr you
might be able to get that way.
Where do you live? I think I have some tungsten sheet that might fit your dimensions.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
FYI, tungsten only has a high brittle/ductile transition temperature. Someone I know has bent thin TIG rods (for heaters for a vacuum metallization
project, of all things interesting) by heating to 300C or so and bending with leather gloves.
There are a lot of tungsten alloys and composites with varying properties. Conductive ceramics may also suffice.
(FWIW, I think he's planning a device with a small, continuous glow discharge.. kind of an odd project, and yeah, smacks of "not showing us
the whole picture", but I digress.)
Tim
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Zr is very hard, I doubt you could roll that. Ir wouldn't work, it forms volatile oxides at those temperatures. I guess that the alloy I
mentioned earlier might not work then.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
The application is the same as the sapphire thread I also have in this forum. I need to make a microhollow plasma cathode as an electron source for a
larger glow discharge. It's a thin insulator sandwitched between two thin electrodes, with a small hole going through the whole thing. Pendulum
electrons between the two conductors start a stable discharge.
I suppose since the heat is concentrated near the hole, I might just need to have a resistant plating on the electrode near around the hole. In my
experiments with copper and mica, both erode very quickly. Alumina would be better, but sapphire is even better than that. I'm guessing pure
tungsten will burn up; as for platinum, it's super expenisve, but if I could use a small amound just for the hole area, and bond it to copper
that helps carry heat away, maybe that would work.
[Edited on 29-4-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Pt isn't really that expensive in small amounts, only about $25/gram.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Can I weld it to copper?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
Tungsten takes a good amount of heat before it starts to react with air in a short amt of time (days is short amt of time). I have used the W sheets
for projects related to what your doing.
Zr is hard, but I can get it into sheets if you want... or at least I can.
Tim, your buddy may have bent TIG rods, which may or may not be pure W, but if you took the rods to a microscope you would find fractures galore. its
not ductile at all, until you make it plastic at temps much higher, but then again, you could say the same about most metals.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
So I guess Pt is the best suggestion this far. But again, how do I bond it to the power leads? I can't solder obviously. Can Pt be welded to
Cu?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Welding will probably result in a brittle intermetallic. Silver solder or brass braze it. Standard jewelry practice, BTW.
Tim
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Zr and W are not suitable for this, they'll simply burn at the temperature mentioned.
The melting point of copper is 1083C, so I can't believe that people are mentioning it here...
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
yes, the MP of copper is below the constrained temp. However its thermal properties are great for getting rid of heat, provided you cool it with
something... like cold water flowing past it.
I use a copper electrodes in my arc melters... which reach temps MUCH higher (6000 C on the cu side). you just have to calculate for volume, time and
how quickly the heat can be transfered to the moving liquid/gas.
I dont think you can braze Pt to copper, as I doubt it would wet the pt. Silver solder might be used, but as soon as heat is applied to said joint,
you have 3 metals, expanding and cooling at completely different rates. The same can be said about welding it, however weldinging it properly it could
be done. But... Since Cu has a lower MP than Pt, you could have had LME in the weld joint, and when the heat is later applied the Cux/Ptx alloy (at
the weld), the Cu may melt within the Pt, not only hosing the weld, but liquid Cu may LME the Pt, through the heating stress. So in effect, it might
hose the entire pieces of Pt by embrittlment. But since Pt still doesnt react with air... eh who cares, its not like your supporting anything with it.
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
an inexpensive alternative... could the experiment be done under inert gas?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Why not just weld a small piece of Pt wire to the foil and connect the end of the wire to something else in a cooler place? You could even just cut a
small strip of the foil and use that as a wire.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
neutrino, a wire is not a good heatsink.
12AX7, I can't silver braze as the silver will melt.
If I made the complete electrodes from platinum, that'd be $500, so I guess so much for that idea.
uber luminal, you mentioned earlier molybdenum and tantalum. However, I read that both metals oxidize at fairly low temperature. You mention some
steels. Can you be more specific? Searching for molybdenum/nickel steels doesn't show any oxidation data for high temperature, so I don't
see how to choose.
I see tungsten/copper and molybdenum/copper used as plasma electrodes, but I don't know if that's just to save on the more expensive
material, and if it's oxidation resistant.
Alumel would seem to be perfect, excet that no one sells it in sheet or foil form...
It says alumel is not resistant to a reducing atmosphere. If there's significant amount of water vapor in the plasma, would freed H ions
possibly create reducing conditions?
Finally, what if I make them out of tungsten and then have it platinum plated? Or is plating likely to erode?
[Edited on 30-4-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
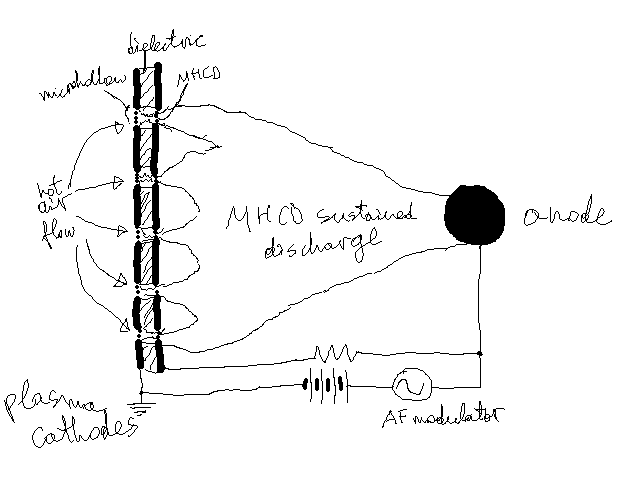
Here's a picture to explain my setup. On the left are five microhollow cathodes -- dielectric sandwitched between planar electrodes.
Thicknesses are about half a mm, as are the microhollow diameters. It operates in air. In my experimentation using copper and mica for the
electrodes and insulator, correspondingly, both eroded very quickly.
Notice that extreme temperatures are only reached around the holes, so if I could somehow bond a more expensive resistant material in only those
areas, it should be fine. But it doesn't seem possilbe. I don't know if even heavy plating would not erode in that area.
[Edited on 30-4-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Your picture is corrupted.
Silver braze does not mean silver, although it could be used. It is an alloy usually of silver and copper, with variable amounts of zinc and/or
cadmium, and is available in melting points ranging from 900 to 1500°F.
Tim
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Firefox, Irfanview, Photoshop, and Paint open the picture just fine.
I melted copper and silver and mixed them for brazing the heater elements for my mantle, and I can still melt it with my lighter.
Hopefully someone can answer my chemistry question from the other post. If plasma makes molecules dissociate into their component atoms, even if I
used a carbon dioxide/water vapor atmosphere (burned propane) instead of air, I'd still have lots of ozone, and the H ions can act as reducers
and wrek alumel? Or will the presence of C make the O more likely to react with the C than the electrode/other O? Basically I'm asking if
there'll be an improvement over air, as of course only a noble gas will be perfect (but I do not have the option of a closed system, and I
can't afford helium tank running off into the outside).
And the platinum plating, would that erode quickly or is that my solution?
[Edited on 1-5-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I didn’t mean to use the wire as a heatsink, rather as a simple electrical contact that could take the heat.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If you are intent on alumel, use air, nothing is less reducing than pure oxygen radicals.
The average condition is what matters, whether there is an excess of H & C vs. O. This is easily set in a burner. Since molecular interaction is
random, your electrodes may eventually wear out....but you were expecting that.
BTW have you looked at conductive ceramics yet? At all? No? Get some graphite and/or silicon carbide and/or a composite with something else like
tungsten (or a PGM) or WC. IIRC, SiC is at least a semiconductor.
Tim
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
You might want to look into inconel, it's a nickel, molybdenum, cobalt (IIRC) alloy which has extremely good wear, abrasion and oxidation
resistance. It's being used in space applications for reentry modules.
Won't be cheap, but I doubt it'll be more expensive than platinum. It's also being used to make crucibles.
[Edited on 1-5-2005 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If this page
http://hcrosscompany.com/metals/inconel.htm
is correct then it is a couple of hundred degrees short in the melting point.
|
|
|
| Pages:
1
2 |