| Pages:
1
2 |
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Hexanitrohexaazaisowurtzitane (CL-20)
Has anyone here attempted the synthesis? The only thing I found is Megalomania's article, but it's not clear that all the reagents can be
acquired without a license. And what is that HBIW listed as a reagent? I didn't find anything on that.
By the way, I'm having a lot of trouble finding any table comparing the power of various energetic materials. Trying to compile one from
different sources sucks, as for example the VoD numbers differ or are given as ranges, etc. For example, it's not clear to me how much more
powerful these new explosives are than, say, NG.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
HBIW stands for 2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-hexaaza-tetracyclo[5.5.0.0.0]dodekan
according to the Project-X vs. Detonator page ( http://pxd.zde.cz/ ) where a synthesis is also given. It basicly is just a condensation of glyoxal and benzylamine. Though making HNIW from HBIW
seems very difficult requiring palladium catalyst and NOBF4 & NO2BF4 for the nitration. Some other methods are available as noted in a thread at
roguesci ( http://www.roguesci.org/theforum/showthread.php?t=4559 ).
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
The roguesci link, now that looks doable at home. Maybe if I find some source of glyoxal I may try it at some point. As for the other link, I
can't read Czech (I was born Bulgarian...), and I doubt my Czech buddy can translate technical terms.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
There are numerous patents on its synth.....you will need a lot of expensive equipment and forbidden precursors 
I think it is so expensive (P2O5, palladium on charcoal-catalyst, very specific glass-equipment,acetonitrile solvent, and and and.....)and
time-consuming it would most likely be a very frustating project.......
[Edited on 18-2-2005 by BASF]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
BASF, do you mean this about glyoxal or HNIW? You don't think that the simple synthesis proposed at roguesci would work?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The method on roguesci if it works will be nearly OTC  . Glyoxal can be made
like formaldehyde or acetaldehyde by passing ethylene glycol over a hot copper wire, and NBK has seen sulphamic acid at home depot, as has a friend of
mine. Only reagent not OTC is the concentrated fuming nitric acid that is needed . Glyoxal can be made
like formaldehyde or acetaldehyde by passing ethylene glycol over a hot copper wire, and NBK has seen sulphamic acid at home depot, as has a friend of
mine. Only reagent not OTC is the concentrated fuming nitric acid that is needed
[Edited on 18-2-2005 by rogue chemist]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I honestly'd like to see a decent ref. on making glyoxal, that doesn't involve gasses and careful regulation of oxygen etc. I read up on
this once, it's hard to prevent further oxidation, how do you suppose it'd easilywork with a Cu-catalysed reaction?
Honestly, I am interested.
[Edited on 18-2-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
From Ullmann(version on FTP)
__________________________________
Among the numerous processes for producing glyoxal, only those using acetaldehyde [75-07-0] and ethylene glycol [107-21-1] as starting materials have
been developed commercially.
From Acetaldehyde. Oxidation with nitric acid was examined by LJUBOWIN as early as 1875 and patented in 1942 [19]. Reaction takes place at ca. 40 °C
and is carried out industrially as a continuous process. Maximum yield is ca. 70 %; selectivity is a function of the relative concentrations of
reagents. After the removal of excess acetaldehyde, the glyoxal formed, which is contaminated with acetic, formic, and glyoxylic acids, is purified by
passage of the aqueous solution through an ion-exchange resin. The solution is then concentrated to a glyoxal content of about 40 %.
Selenium oxide [7446-08-4] is more selective than nitric acid, and the yield is ca. 84 %; the selenium can be recycled by oxidation with hydrogen
peroxide [20]. This process has not been carried out on an industrial scale.
From Ethylene Glycol. The gas-phase oxidation of ethylene glycol by atmospheric oxygen in the presence of dehydrogenation catalysts (metallic copper
or silver) represents the basis of the Laporte process [21] and has been used in several industrial production processes. Reaction occurs between 400
and 600 °C; the yield is 70 – 80 %. The main impurity is formaldehyde [50-00-0] , whose subsequent separation is difficult. This reaction has also
been carried out in the liquid phase and under irradiation.
Other Processes . Ethylene can be oxidized by aqueous nitric acid in the presence of palladium [22] , by atmospheric oxygen, or by selenium oxide
deposited on silica [23]. Glyoxal may also be formed by oxidation of acetylene [24] or benzene [25] with ozone. Ethylene oxide has been proposed as a
substrate for oxidation. Although oxalic acid and its derivatives can be reduced to glyoxal, these processes have not been developed further.
[19] I. G. Farbenindustrie AG, BF 885 931 (1942).
[20] Air Liquide, FR 2 038 575, 1969 (J. P. Zumbrunn).
[21] Laporte Chemicals, GB 1 272 592, 1963 (B. K. Howe, F. R. Hary, D. A. Clarke).
[22] BASF, DE 1 166 173, 1962 (R. Platz, W. Fuchs); DE 1 231 230, 1964 (R. Platz, H. Nohe).
[23] E. Costa Novella, Ann. Quim. 68 (1972) no. 3, 325 – 332;Chem. Abstr., 77, 113 760 f.
[24] Imperial Chemical Industries, GB 1 071 902, 1965 (R. A. Rennie).
[25] Inmont Corp., US 3 637 860, 1968 (W. P. Keaveney, J. J. Pappas).
-------------------------------------------
I have no idea how easily this work, ideally it would be as easy as formaldehyde for methanol but now you bring up the issue of over oxidaton, which I
was unaware of. When I posted that I had not done any indepth research, just what I found in Ullmann's. Well this weekend I will be doing some
looking into of the references cited. Hopefully there is something feasible. Are the references above any of the same ones you have loked into?
Oxidation of acetylene with ozone seems...well...interesting
[Edited on 18-2-2005 by rogue chemist]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Is it possible to purchase it instead, without any special license? I don't see it on any restricted lists.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I didn't have any trouble buying glyoxal through a chemical supplier ( they only sell to companies, but I think most of us know someone who owns
a small one-man company ).
Regarding the HBIW --> HNIW route if you choose to go that way; the difficult part is the debenzylation of the isowurtzitane skeleton, not
nitration. It is true that in the original China Lake method they used NOBF4 and NO2BF4, but within the last 3-4 years a number of processes involving
only HNO3 and optionally H2SO4 have been developed.
They have been published both in the form of patents and in peer reviewed journals such as Propellants, Explosives and Pyrotechnics.
Edit: Let me just clarify: Most of these new methods concern the nitration of debenzylated precursors such as TAIW ( tetraacetylisowurtzitane ) or
TADB ( tetraacetyldibenzylIW ). The only places I have seen a method that didn't need Pd-catalysis for preparing the nitration substrate are
those mentioned in my thread on roguesci.
[Edited on 18-2-2005 by Microtek]
|
|
|
deiki
Harmless

Posts: 8
Registered: 14-3-2006
Location: a random manifold
Member Is Offline
Mood: chaotic
|
|
Does anyone know if the Ebele process with glyoxal instead of paraformaldehyde ( ammonium nitrate + glyoxal + Ac2O ) and some pressure has been
discussed or attempted  ? If it doesn't yield HNIW, maybe it could form another
interesting structure or ... nothing ? If it doesn't yield HNIW, maybe it could form another
interesting structure or ... nothing 
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
2 stage synth of Cl-20 from a primary amine
( Hexanitrohexaazaisowurtzitane )
http://www.freepatentsonline.com/7279572.html
Related download posted by kmno4
Methods of synthesis and properties of hexanitrohexaazaisowurtzitane
http://www.sciencemadness.org/talk/viewthread.php?action=att...
The original patent _
http://www.freepatentsonline.com/5693794.html
.
|
|
|
Nixie
Hazard to Others
  
Posts: 490
Registered: 12-12-2006
Member Is Offline
Mood: ?
|
|
I'm surprised at the lack of posting here. No hobbyist tried to DIY this yet?
\"Good is a product of the ethical and spiritual artistry of individuals; it cannot be mass-produced.\" --Aldous Huxley
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Here is a ChemDraw outline of the SNPE reaction sequence. They use a variety of different amines but the nitration examples are both based on the
allylamine condensation product with 40% aqeous glyoxal followed by conventional mixed acid nitration. They state a 40% yield for the N-allyl
intermediate but I could not find anything on the yield in the nitration step.
[Edited on 23-7-2008 by Ritter]
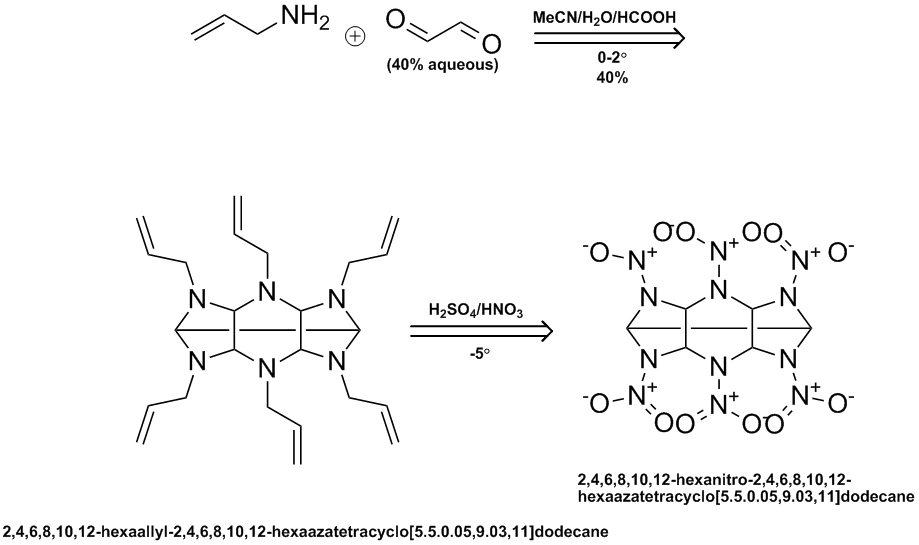
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Here's a ChemDraw summary of the process worked out by Thiokol & later Cordant. See the following US patents:
5723604
5739325
6147209
7129348
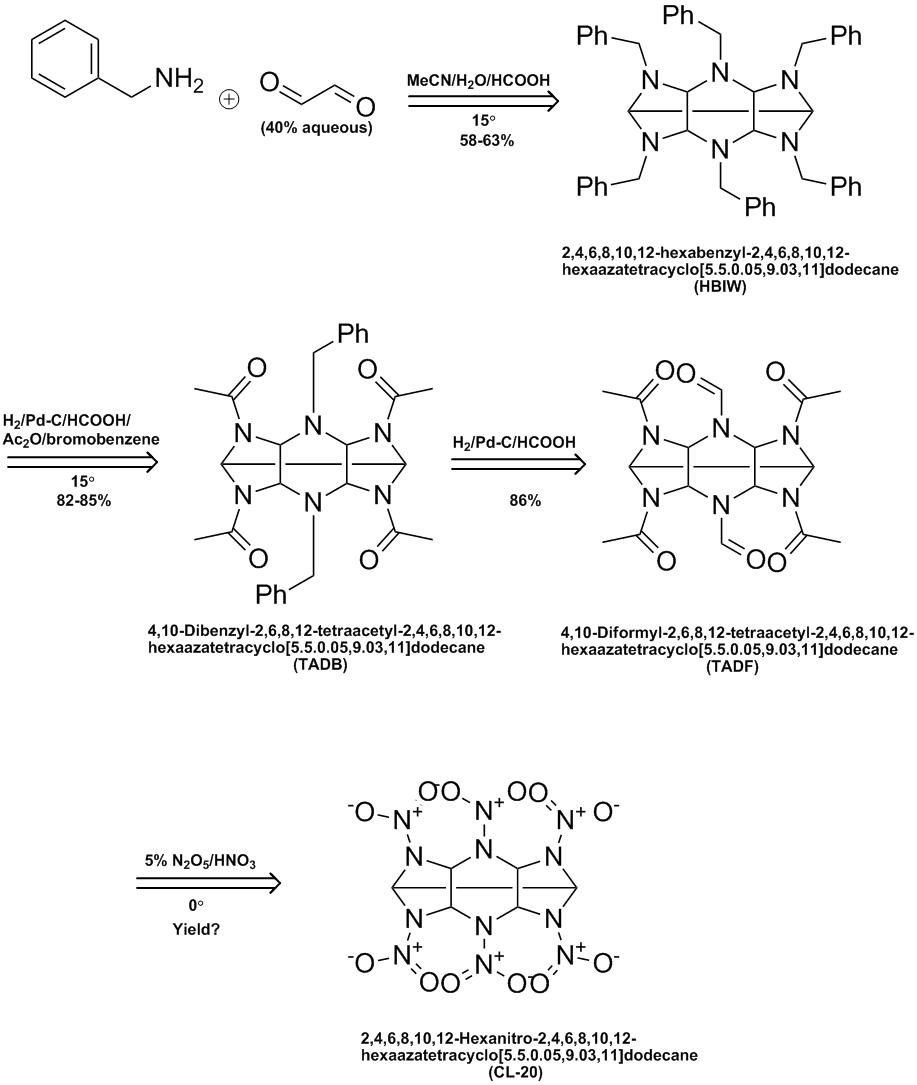
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Here is a variation of the CL-20/HNIW process disclosed in US6391130 (see http://www.pat2pdf.org/patents/pat6391130.pdf). In this vasriation HBIW is converted to TADB which is then converted to TADH which is then
nitrated with mixed acids to give the explosive.
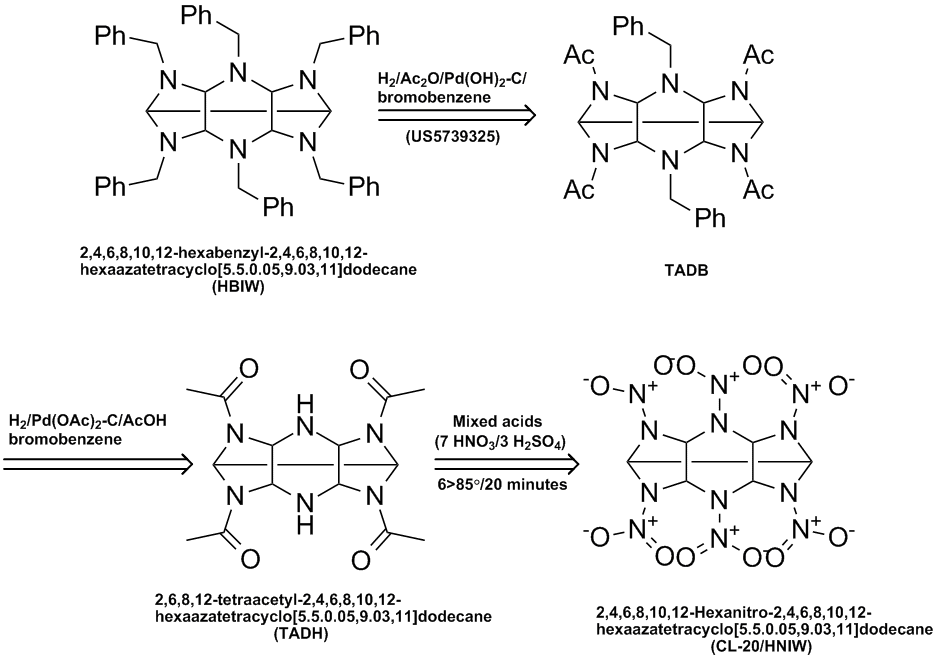
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Just a thought on this thread: anyone planning to duplicate any of this chemistry should check the original patent & other literature references
before starting. There are a number of patents describing variations of the different process steps & the amateur experimentalist should first
convince himself that he has a complete & clear picture of the chemistry prior to proceeding. While creating the ChemDraw graphiocs files was fun
& practice for me, they should not be used as primary references.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by BASF
There are numerous patents on its synth.....you will need a lot of expensive equipment and forbidden precursors |
I believe Ac2O is on the forbidden list. It is an amphetamine precursor.
| Quote: | I think it is so expensive (P2O5, palladium on charcoal-catalyst, very specific glass-equipment,acetonitrile solvent, and and and.....)and
time-consuming it would most likely be a very frustating project.......
[Edited on 18-2-2005 by BASF] |
I would reccomend a fully equipped lab for this chemistry. Plus a lot of skill.
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Actually there are quite a few threads on this substance around.
Some even show what it really looks like structurally.
The drawings above are correct but manage to be correct in a way that conveys little information. The molecule is not flat. The C-C cond between the
two 5-membered rings is not ridiculously long.
As you can see from the sketch attached, the piperazine ring is in an inverted boat conformation and the two 5-membered rings parallel each other.
Sometimes the patent drawings are inferior. There is no need to be slavishly devoted to them when more intelligent and informative representations are
available.
[Edited on 27-7-2008 by Sauron]
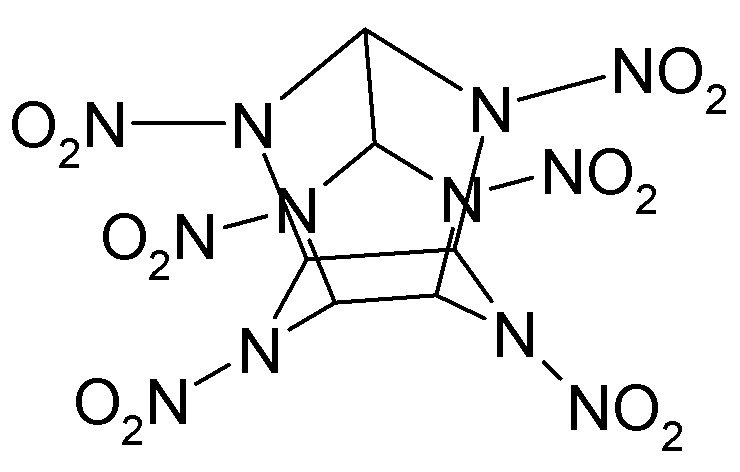
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The (perceived) aesthetic differences between 2 different extended structural representations of the same fused ring system misses the point of my
posts. I was intending to convey the materials flow & reaction chemistry with a number of variations between patents.
As a further indication of the difficulty involved in this synthesis, attached is the lab-scale setup for this synthesis.
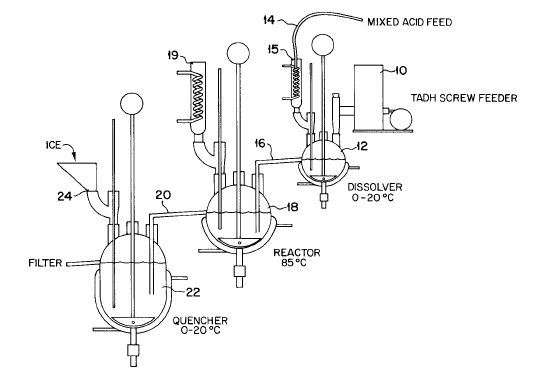
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ac2O is not on any "forbidden list" whatever that is. It is not a DEA scheduled controlled substance and is in no way a precursor to amphetamine(s).
It may be one of one of the DEA lists of chemicals they watch, mostly because of its role in the production of heroin from morphine (main alkaloid in
opium). However this is primarily a problem in heroin producing regions. The US is not a heroin producer but a consumer.
Acetic anhydride is widely used in industry and in research.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Ac2O is not on any "forbidden list" whatever that is. It is not a DEA scheduled controlled substance and is in no way a precursor to amphetamine(s).
It may be one of one of the DEA lists of chemicals they watch, mostly because of its role in the production of heroin from morphine (main alkaloid in
opium). However this is primarily a problem in heroin producing regions. The US is not a heroin producer but a consumer.
Acetic anhydride is widely used in industry and in research. |
I suggest we start another thread as this is OT here.
Ac2O is used, for example, in the Ube phenylacetone synthesis outlined in JP59152342. No English equivalent issued but I have the English abstract
from the EPO.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Starting yet another worthless thread is your answere to everything, Ritter.
Where's the "forbidden list"?
Tracked chemicals are by definition NOT forbidden.
If they were forbidden there would be no sales to track.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Starting yet another worthless thread is your answere to everything, Ritter.
Where's the "forbidden list"?
Tracked chemicals are by definition NOT forbidden.
If they were forbidden there would be no sales to track. |
You are off topic again.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Why not just admit you were wrong?
Because you were. Telling you so is not off topic, much as you would like to deem it so.
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |