| Pages:
1
2
3
..
5 |
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Nicotine Extraction and purification
This has been discussed and dropped for lack of information many times over the years so I've been reopening the discussion on various forums
with the hope of actually managing to do it. I want to extract nicotine from tobacco and purify it.
Now, before the more annoying......er uh, helpful of you start telling me how it's easier to just smoke the darn cigarettes, how toxic it is, how
it can be absorbed through the skin, how it can cause cancer, etc. I've already experimented with the material and I know exactly how to handle
it. One can expound about how it's the most poisonous substance known (untrue), it has no treatment for overdose (untrue), or how the LD50 in
rats is 50mg (true), but that isn't helpful. And, no I'm not a terrorist or murderer that wants the stuff to kill people with. That's
mostly a bunch of TV supported hype anyway.
I've tried acid base extractions with naptha/water, choloroform/water, toluene/water all have resulted in something, but not much, and very
unpure. I tried steam extraction, but this results in a bunch of water and very little alkaloid.
Does anyone know or have a way to get the solubility of nicotine in various solvents? Or maybe one of the easy derivitives like nicotine sulfate,
hydrochloride, citrate? No need suggesting a web search, I've done that ad nauseum. The libraries I have easy access to have practically nothing
to offer.
No, removing it from the patches is not a good option. The goo they mix it into is hard to deal with. Especially with the small quantities involved.
The inhalers are easy, but too darn little for too much money. I don't have access to the nasal devices. The lozenges have similar problems in
that the additives to slow the intake are impossible to remove; they're also expensive.
I have discovered a number of things. Don't start off with water, the tobacco swells up and absorbs the water leaving you with tobacco jelly that
is tough to deal with.
Nicotine disolves in everything, additionally, it seems to dissolve anything as well.
Being water soluble it is tough to get out of water, but forming a hcl or sulfate salt will move it to a non polar.
Every extraction seems to loose a bunch of it. Even with extremely heavy salting.
Most of the patents don't work as advertised, they lead to mostly blind alleys.
The schoolbook extractions don't work well, there are so many washes and extractions that you run out of nicotine before you finish. The
mechanical losses are too big.
Kerosene (yuck) is a really efficient extraction solvent.
Suggestions? Solubility data?
I already have the Merck entry (not very helpful) and have reviewed about 700 patents.
In answer to a question that I’m sure will come up since it has in every single thread I've found about it. About three years ago I tried to
quit smoking (for the 17th time) and used the patches and inhalers. The patches gave me trouble and the inhalors were too darn expensive for a tiny
hit. Did you know they allow one to use up to 40 of these things a day. So I reverse engineered the inhalers and created my own that I can refill all
I want for 1/1000th of the cost. I've also created nasal sprays, lollipops, lozenges, etc. none of them FDA approved.
Now before someone starts advising me about the risks of nicotine, keep in mind that for three years I've controlled the dosage myself and
handled this substance without mishap. The reality is that it's not nearly as toxic as one would be led to believe based on the hype out there.
My methodology is more sophisticated and safe than buying X from a guy on the corner or in a booth at a bar.
Now, I want to extract it myself rather than order it from chemical suppliers since the patriot act may flag me as a suspicious person. Additionally,
a lecture on why don't I just wean off the substance will fall on deaf ears since I've been trying that for three years as well. We
don't all have the same makeup and it seems to be harder for me. But I don't smoke anymore and no one cares if I suck on a lollipop or have
a lozenge in my mouth. Snuff and chewing tobacco are not options, they’re as dangerous and offensive as smoking.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Remember that it’s also used as a pesticide. You might be able to get it from that. There is a thread at RS about this here, if it helps.
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Thank you for the pointer. I went and looked at that thread and it is typical of all the threads I've looked over the years.
Someone asks how is it possible and then they spend days explaining why they want to do it. While people rail at them about how dangerous it is and
how they shouldn't do it.
I've been lurking on this board for a few weeks and I've found this board really good and the folks here not as prone to attack as many
other places.
However, the thread on RS did point out a problem with my posting. (actually 2 but I'll get to the second one later). I didn't tell you what
I've already found out.
Like I said most of the patents just don't work; at least in smaller quantities. For example to create Nicotine Sulfate the industrial process is
to percolate a non-polar through tobacco and then pump it into the bottom of a container of dilute sulfuric acid. As the non polar rises (note the
density requirement here) it releases some of the nicotine to the acid to produce nicotine sulfate in the water. The non polar is pumped back into the
percolator.
This process proceeds until the dilute sulfuric is neutralized, and behold, you have nicotine sulfate in water. This used to be known as Black Leaf
40. 40% because at concentrations over that it would precipitate out.
However, doing this at home is tough. Maybe a fuel pump and a coffee pot or something could be employed, but it has evaded my skills so far. I have
succeeded in making some interesting messes though.
Another method is to extract the tobacco with MEK then extract with acidic brine If you basify the brine and heat it, a separation happens that is
purported to be a separate phase of nicotine. Well, they lied and didn't consider the solubility of MEK in water. What you get is MEK and junk
floating on top that is mostly sugars, fats and such. I wasted more than a month on that method.
An extraction of tobacco with kerosene followed by an extraction with acidic water rinsed with naptha, ether, naptha. Basify, salt and extract with
naptha on evaporation will give you pretty pure nicotine, but the washes worry me about losses. Also, I can't seem to get rid of all the naptha.
There always seems to be a bit of it in there.
How the heck do you evaporate the solvents from a tiny quantity of liquid product.
Final purification? Don't get enough of this stuff to distill. Even so, I'm worried about degradation due to heat. I could possibly steam
distill it, but how the heck do I get it out of the water?
Now, to the mistake I made above. If you acidify it it will force most of it into the water, not the non-polar! I can't believe I messed that up.
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Oh, thanks for the pointer to insecticide. However that form of insecticide has been discontinued in the US for several years. There's tiny
amounts in a few products, but not enough to try to use. There (apparently) is only one product left that has nicotine sulfate in it and that is only
commercially available for hothouse fumigation.
I tried to get it through chemical suppliers. What a joke, they all thought they should report me to homeland security except for one that was willing
to sell it to me for U$5 a gram in 100g quantities. Quite a mark up from the U$86 dollars that acros sells it for. That supplier would probably have
called homeland security as soon as the check cleared.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
You could try a butane extraction like I've seen on the Internet for cannabis...
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Regarding butane. I did this one with a piece of pvc pipe and about 100g of tobacco. The extraction worked exactly as described for THC, but it
produced a grease that wasn't active. I think I took out oils and such and left the nicotine behind. Tried this twice and got the same results.
There is a possibility that I haven't tried. Nicotine is held in the tobacco leaves as various salts as well as the freebase. If one were to
dampen the tobacco, add lye and mush it all together it could be that this process would release more of the freebase to be liberated by the butane.
The commercial process for this uses CO2, but I don't understand how to do that at home.
I did find a french procedure that used picric acid to form a picrate with nicotine that (apparently) precipitates from water. I haven't tried it
since I would then have to find a way of disposing of the picric acid.
If I could find a salt of nicotine and a solvent it was insoluble in.......
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
I see you've already checked usenet, so I won't regurgitate what I read there. The zinc chloride double salt thing sounded interesting,
though. Your best bet would be to rig equipment to do the industrial procedure. What's so hard about a pump, anyway?
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
From <A HREF="http://bcis.pacificu.edu/~polverone/alkaloids-jbig2.pdf">The Plant Alkaloids</A>:
| Quote: | Nicotine, C10H14N2. The pure alkaloid is a colourless oil, b.p. 246.1 °/
730-5 mm. It can be purified
through the crystalline zincichloride, B,ZnCl2,2HCl,H20, the regenerated
base being distilled under reduced pressure (20-40 mm.) in presence of
nitrogen or hydrogen. It distils unchanged in a current of steam and is
readily soluble in alcohol, ether or light petroleum. The behaviour of
nicotine with water has been studied by several workers.19 It is miscible
in all proportions with water below 60° and above 210°; at intervening
temperatures soluble hydrates are not formed and miscibility is limited.
According to Kelly et al.19 an azeotrope is formed, which contains 2.45 per
cent, of nicotine and boils at 99-6°/760 mm. The salts are readily soluble
in water, do not crystallise easily and are dextrorotatory.
...
The acid d-tartrate,
B . 2H2C4H4O8 . 2H2O, m.p. 88-9° (hydrated), and
the neutral d-tartrate, m.p. 68-5° (hydrated), , both
crystallise from alcohol on addition of ether. The dipicrate,
B . 2C6H2(NO2)3OH, short yellow prisms, m.p. 224°, and the tetrachloriodide,
22 C10H14N2.2(HICl4), orange prisms, m.p. 150° (dec.), are
characteristic. |
That's probably nothing new to you, but it does confirm that there are limited options for purifying nicotine. Try forming the dipicrate. Picric
acid can easily enough be disposed of by conversion to its ammonium salt and burning. There is little in risk in disposing of limited amounts of
picric acid this way.
PGP Key and corresponding e-mail address
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Isopropylamine should be a good alkaline extraction solvent for free alkaloids. I believe the boiling point is in the same range as that of ether.
Diisopropylamine boils in the 70's if you want something that evaporates more slowly.
Either one should be fairly simple to make from acetone, an ammonium salt, and a dissolving metal such as amalgamated aluminum, zinc dust, nickle salt
treated aluminum or zinc, magnesium turnings, ect.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Gattermann-Wieland, will scan...eventually
Commercial tobacco extract (300 c.c, d. 1.8), which can also be prepared by concentration of the faintly acidified dilute extract to be had in any
cigar factory, is made strongly alkaline with concentrated sodium hydroxide solution. Steam is passed through the hot solution and the free nicotine
bases pass over. About 1.5 L of distillate are collected, made faintly acid to Congo red with solid oxalic acid (a weighed quantity), and concentrated
to a syrup. When the syrup cools nicotine oxalate contaminated with some ammonium oxalate separates. The crystalline sludge is transferred to a
separating funnel into which is poured rather more potassium hydroxide solution (1:1) than corresponds to the oxalic acid used. Heat is developed, and
after some time the crude nicotine rises to the surface as a brown oil, which is separated from the cooled mixture by repeated extraction with ether.
The concentrated ethereal solution is dried with a few pieces of solid potassium hydroxide and then the ether is evaporated. The residue is
fractionally distilled in a vacuum from a small Claisen flask. Since rubber stoppers are attacked by nicotine air-tight corks are used instead.
By repeated distillation of the higher boiling fraction the pure base is obtained as a colourless liquid of boiling point 114°/10 mm., 120°/14 mm.
At atmospheric pressure nicotine boils without decomposition at 240°. The yield varies from 4 to 6 g.
The specimen turns brown very soon if exposed to the air, and must be kept in a sealed glass tube.
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
I'm in a densly populate area and a little reluctant to deal with picric acid (no experience with that substance). The oxalic acid idea
doesn't sound bad at all, that may be my next try, but I'll have to figure out how to get it into alcohol.
Alcohol is as bad as water in the extraction, the tobacco swells up and clogs everything.
The problem with a pumped percolator is that it clogs up. I haven't found a way to screen the tobacco without stopping fluid flow. This stuff
just falls apart when made basic to release the salts of nicotine into a freebase and clogs up everything.
I also tried vaporization. Heating the tobacco to 200C with a cold finger. This condensed some acrid water, but not much else.
Oh, I've tried several different sources of tobacco, Marlboros, cigars, pipe tobacco, and even the dust used in organic gardening. It seems that
the cheaper it is, the easier it is to extract.
Any clues on how to isolate the .hcl salts or sulfates?
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Just to let you know that I take your suggestions and ideas seriously, I looked really closely at the Gattermann-Wieland isolation above.
I thought this was a great possibility until I finally discovered that the "commercial tobacco extract" was actually referring to the
nicotine sulfate insecticide that used to be sold all over the world. This is 40% nicotine sulfate and it would be easy to get product out of
something that concentrated. The idea of boiling tobacco down to a slurry was one of the first things I tried, but nicotine would escape with the
vapors.
However, the oxalic acid is still a real possibility.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Assorted Nasties by Harber has a step by step cookbook method for extracting nicotine, the basic outline is to thoughly dry your starting
material, powder it, a lenthy process of heating with lime and water and letting sit for periods, filtering, boiling, filtering, boiling, and finally
distillation, followed by a liquid/liquid extration of the resulting azeotrope and evaporation of the ether used for the extration to yield "a
very good grade of nicotine."
[Edited on 2/18/2005 by BromicAcid]
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Thanks BromicAcid. I've looked at about 7 of the water methods an they all have the same problem absorption into the tobacco. Let's say you
take 100 grams of tobacco and add 300ml of water to it with some NaOH. You will get swollen tobacco that won't release the water. It can't
be filtered or screened. You can wrap it up in a rag and squeeze which will give you back around a hundred ml of dark solution that still won't
filter.
If you try to extract this it will form an emulsion that just won't separate. I've left these setting for days with no luck.
Using a brine to soak helps some, but there is little solubility in brine (that's, after all, the point) and I don't believe the extraction
actually works.
I've had the best luck with a non polar solvent for the first extraction. Much less swelling an it can be filtered....sort of.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
How about just steam distilling from tobacco in alkaline water?
You might want to have a really tight distillation setup and good ventilation.

|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Why not just use a lot of acidic water and then boil it down?
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
I took 50 grams of tobacco and steam distilled 1.5 liters of water through it. I then acidified and reduced the volume to around 100ml and extracted.
Nothing active came out.
I then took the same tobacco (I figured something had to be in there) and based it to ph14 in 100ml of water. Yes, it turned into goo and distilled
another 1.5 liters of water through it. Distilled that down to around 150ml and extracted. Got some actives, but it was bound in a large amount of
black goo. I acidified the goo with hcl and washed with ether then based and extracted. I got maybe a drop of dark brown oil that was just barely
active.
I have not tried the idea of using a LOT of water for extraction. The idea I'm going to try is to extract 100g of tobacco with about a gallon of
basic water ph>14. This will give a messy black liquid. This I'll screen a couple of times and then press in a coffee can with holes to get as
much of it as possible.
Then acidify it to ph < 3 and distill it all through a column to keep the solids and whatever else remains. I'll then wash the residue with
ether before a normal acid base extraction. Save the ether though in case nothing comes out.
this will be a pain since distilling that much water takes time in a small setup, but it should prove that I can get something out.
Any suggestions would be welcome, I'm going to start this tomorrow.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by Cheapskate
I took 50 grams of tobacco and steam distilled 1.5 liters of water through it. I then acidified and reduced the volume to around 100ml and extracted.
Nothing active came out. |
If you add acid you proptonate it, the result is soluble in water and insoluble in non-polar solvents. What phase did you save? If you saved
non-polar phase there is wonder that you got nothing.
Authors seem to have limited lab-experience, but the method nontheless could give you some idea of how this A/B extraction could be done (what layers
to save and so on):
http://mapage.cybercable.fr/awebster/nicotine/method.htm
[Edited on 19-2-2005 by Sandmeyer]
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Na2CO3, or even NaHCO3, should be good enough as a base. for the initial hydrodistillation.
If you start with the pH too high, you are going to get all kinds of degradations, condensations, hydrolysis reactions.
Use fractionating column, slowly distill H2O/nicotine azeotrope, make alkaline, extract,THEN concentrate.
[Edited on 19-2-2005 by Eclectic]
|
|
|
Cheapskate
Harmless

Posts: 27
Registered: 3-2-2005
Member Is Offline
Mood: Just fine, than
|
|
Sandmeyer, Thanks for the pointer to the workup. I found that about a month back and read it in great detail. Notice that the kids didn't get
anything substantial. I loved the part about having to keep going back for more solvents.
Let me assure you, I've had the same problem. When one is extracting directly from the natural products there is a TON of stuff that you have to
deal with. The fats turn into soap and allow normally non-polar materials into the non polar solvents. So you can wash essentially forever and just
keep getting trash out.
It may be that Eclectic is right, that I'm destroying the nicotine with a solution that is too basic, but I can't tell. There may well be an
azeotrope of nicotine and water, but I can't tell that either. If you distill water and tobacco, how the heck do you tell if you've run
enough water through the system. Like I said, I put 1500ml through a hundred grams and it was still coming over yellow. ...and I didn't get
anything.
There were some lorillard papers that indicated that steam distillation under high pressure was the common way of commercial water extraction. That
would explain my lack of success. However, my personal experience has been that nicotine is very soluble in water so a straight water extraction seems
to be the best first step if I can somehow get the darn water back, it just wants to stay in the tobacco.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Re possible uses of nicotine, which consists of a N-methyl-tetrahydropyrrole ring attached at its 2-position to the 3-position of a pyridine molecule:
as well as an insecticide, it can be also oxidized with concentrated HNO3 (and some other oxidants) to obtain nicotinic acid, or pyridine-3-carboxylic
acid, which is vitamin B1 (which is incorporated into the coenzyme nicotinamide adenine dinucleotide.).
Because of the polarity of the molecule, the best solvents for a nicotine solvent extraction should be non-acid polar organic compounds, especially
aromatic ones like pyridine.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
John, yes, nicotine can be oxidized to niacin and is incorporated into NAD, but niacin is NOT, I repeat, NOT Vitamin B1. That's thiamine. Just
wanted to point that out.
Cheapskate, have you tried extracting with ligroin?
sparky (^_^)
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
For your own personal use in very small quantities, have you considered using an expresso machine for the initial extraction?
You might have to do a run once a week or once a month, but nicotine oxidizes very easily anyway. You could acidify the initial extraction water with
citric, abscorbic, or acetic acid to avoid machine damage.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by Cheapskate
It may be that Eclectic is right, that I'm destroying the nicotine with a solution that is too basic, but I can't tell.
|
I can see nothing in the structure of nicotine suggesting it can be "destroyed" by a base (nucleophile).. The only site where a
hypothetical nucleophilic attack might take place in nicotine is the C=N fragment of the pyridine ring. Not only is this site not sufficiently
electrophilic, but if the attack was to happen it would destroy the aromacity of the pyridine ring - a highly unfavorable situation. In other words
this would not happen, not even a organo-metallic reagent would add like that , let alone the OH...
| Quote: | | Originally posted by Eclectic If you start with the pH too high, you are going to get all kinds of degradations, condensations, hydrolysis
reactions. |
Really? Cool...
[Edited on 20-2-2005 by Sandmeyer]
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Side reactions due to everything other than nicotine that is in the tobacco.
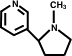
You don't think the pyrrolidene ring is subject to attack? I'm thinking that some of those carbons could give up H+ .
[Edited on 20-2-2005 by Eclectic]
[Edited on 20-2-2005 by Eclectic]
|
|
|
| Pages:
1
2
3
..
5 |