runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
N-bromo Succinimide
Just a question on synthesis of this compound.....
Could this be achieved by the following scheme, see if im on the right track:
1) Succinic acid (HOOC-CH2-CH2-COOH) heated with ammonia to form succinimide (slight variation on --> http://themerckindex.cambridgesoft.com/TheMerckIndex/NameRea...).
2) As per rhodiums synthesis of NBS:
1.62mol (160g) succinimide is dissolved in a mixture of 1.60mol (64g) NaOH, 300g crushed ice and 400ml water. Cool the mixture in an ice bath, and add
85ml (1.65 mol, 264g) Br2 at once while stirring violently. Stir for two more minutes, then filter the precipitated product and wash with ice water.
Dry in a desiccator. Yield 75-81%.
Would this work?
Thanks.
-rlr
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Yes, you're right. You can also heat succinic acid with urea to 175 °C to get your succinimide. In Gattermann 'Die Praxis des Organischen
Chemikers' (don't know if it’s translated) there are preparations of succinimide from succinic acid and ammonia and succinic acid and
formamide. The NBS-synthesis is described there too.
Does anybody tried to substitute NBS with OTC pool brominators? It might even work better than NBS, like the example of NCS and TCCA
(for water-disinfection) shows. It only depends from what those pool brominators are made of. Because they giving bromine very slowly away, I am
optimistic, that they might be as selective as NBS.
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
OTC brominating agents
Common OTC pool brominators consists either of BCDMH (1-Bromo-3-chloro-5,5-dimethylhydantoine => product: Aquabrome) or DBDMH
(1,3-Dibromo-5,5-dimethylhydantoine => product: Albrom).
DBDMH:
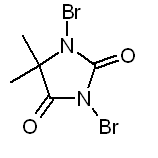
BCDMH reacts with water to form both hypochlorous and hypobromous acids. DBDMH forms only the hypobromous acid and should be sufficient for
bromination. As I am in hurry, I would be glad if someone could check DBDMHs ability as brominating agent.
I think, this gives a good summary of possible OTC brominating agents.
~Mephisto
|
|
|
enima
Hazard to Self
 
Posts: 98
Registered: 1-12-2004
Member Is Offline
Mood: No Mood
|
|
Yup, it can brominate. Works well only on activated rings..
Tetradehdron Letters, Vol. 38. No. 25 pp. 4415-4416, 1997
anisole is brominated with a 85% yield (18 hours)
1,3-dimethoxybenzene is brominated with a 98% yield, (18 hours).
2-methylphenol is dibrominated.
Anisaldehyde, toluene and 2-picoline fails bromination.
more info in the article... have no time..
|
|
|
UpNatom
Harmless

Posts: 42
Registered: 2-9-2004
Location: UK
Member Is Offline
Mood: No Mood
|
|
OTC halogenation
Check it out:...N-Chloro and N-bromosaccharins: valuable reagents for halogenation of electron rich aromatics and cohalogenation of
alkenes
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S...
NCSac and NBSac are synthed from sodium saccharin and KBr/KCl and oxone.
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Merci
Nice. Just wanted to say thank you for both references!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Great!
This reagent is a great advancement in OTC chemistry. Thanks for the info. Hopefully this product can be located OTC in EU as wellt. Bye, bye NBS...
|
|
|
UpNatom
Harmless

Posts: 42
Registered: 2-9-2004
Location: UK
Member Is Offline
Mood: No Mood
|
|
?
Anyone have the iodide/iodate method formerly at www.rhodium.ws/chemistry/aromatic.iodination.iodide-iodate.h...
(taken from Tetrahedron Letters 44(27), 5099-5101 (2003))
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
there are plenty of rhodium mirrors around, search for them and you can easily find the page you want...
-rlr
|
|
|
UpNatom
Harmless

Posts: 42
Registered: 2-9-2004
Location: UK
Member Is Offline
Mood: No Mood
|
|
A U2U to another
.,.member at this board has prompted me to post what I should have included earlier. Euroscientists...where you see oxone, insert virkon.

|
|
|
Caffinehog
Harmless

Posts: 9
Registered: 3-3-2005
Location: Under a rock somewhere
Member Is Offline
Mood: Unstable
|
|
Just remember that this stuff is good for radical reactions, too.
I don\'t understand what you are saying, but I agree with every word of it!
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
| Quote: |
Just remember that this stuff is good for radical reactions, too.
|
Truly. For the times peroxides and AIBN are inaccessible... 
sparky (^_^)
[Edited on 6-3-2005 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
This idea of brominating with DBDMH was just a fast thought. However, in very rare cases DBDMH is even more efficient then NBS. For example to
dibrominate piperonylic acid (as I said, these cases are very rare). As I scanned two articles about DBDMH-bromating to inform myself about its
possibilities, I can also share these articles:
[color=darkred]Tetrahedron Letters, Vol. 34, No. 6, pp. 931-934 (1993)[/color]
Title: N-Bromosuccinimide, Dibromodimethylhydantoin in Aqueous Base: A Practical Method for the Bromination of Activated Benzoic Acids
Abstract: A new bromination method employing NBS or dibromodimethylhydantoin in aqueous base is described for the synthesis of
3-bromo-2,6-dimcthoxybenzoic acid (1) and other monobrominated alkoxy benzoic acids.
[color=darkred]Tetrahedron Letters, Vol. 38, No. 25, pp. 4415-4416 (1997)[/color] - found by enima
Title:1,3-Dibromo-5,5-Dimethylhydantoin, a useful Reagent for Aromatic Bromination
Abstract: 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) is a useful and easy to handle reagent for bromination of various aromatic
derivatives substituted with electron donating groups. In the presence of trimethylsilyltrifluoromethanesulfonate, DBDMH showed increased reactivity,
and in one case, the reaction followed another pathway, suggesting an alternative mechanism.
BTW: Has anybody seen DBDMH containing products in Europe? It seems that the Albemarle products 'XtraBrom' and 'Albrom' are only
available on the US-market.
****
Both articles are attached.
Attachment: Both.Articles.rar (207kB)
This file has been downloaded 849 times
|
|
|
enima
Hazard to Self
 
Posts: 98
Registered: 1-12-2004
Member Is Offline
Mood: No Mood
|
|
Nice find Mephiso. I wonder how well the DBDHM would work on activated aldehyde groups. Those are very nice yields for the bromination of benzoic
acids. I must say very impressive.
|
|
|
UpNatom
Harmless

Posts: 42
Registered: 2-9-2004
Location: UK
Member Is Offline
Mood: No Mood
|
|
| Quote: |
BTW: Has anybody seen DBDMH containing products in Europe? It seems that the Albemarle products 'XtraBrom' and 'Albrom' are only
available on the US-market
|
Yes indeedy...Maxibrom and Allbrom are what you're looking for (possibly other brands as well but I don't have time to
check). Btw the first hit on google UK for either of those two products gives a company which also sells an item called "Shock II" which you
may find interesting/useful 
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
UpNatom: I searched for the Dibromdimethylhydantoine, while Allbrom and Maxibrom contain Bromochlorodimethylhydantoine. There
is a chance that the bromochloro-mix works too, as Br is a better substituent than Cl, but to be on the safe side I am looking only for the chlorine
free substance.
|
|
|
UpNatom
Harmless

Posts: 42
Registered: 2-9-2004
Location: UK
Member Is Offline
Mood: No Mood
|
|
My apologies Mephisto, you are correct in that Maxibrom contains Bromochlorodimethylhydantoin as well as the dibromo compound however allbrom
(according to the manufacturers MSDS) contains only the dibromo compound.
You can find the msds here: http://www.aldenleeds.com/html/allbrom.html
[Edited on 16-3-2005 by UpNatom]
[Edited on 16-3-2005 by UpNatom]
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Oh, sorry - you're right. Seems like I opened the Maxibrom-PDF twice instead of the Allbrom-PDF, my fault. And thank you for your search of
course.
~Mephisto
|
|
|