kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Boric acid from commercial sodium perborate >30%
Hallo to all,
how to make boric acid from commercial 30% sodium perborate?
Thanks for help!
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Add concentrated HCl to a solution, boric acid will precipitate.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Thanks!!
Hallo Bromic,
thanks for help!
Can you be more linear?
I must add conc. HCl (I've 30% HCl) to a solution of sodium perborate powder in water? Won't sodium perborate react with H2O to give H2O2?
Thanks for help! 
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Sodium perborate, like sodium percarbonate varies in having the -O-O- linkage within the molecule, or having a peroxide molecule of crystalization, I
believe. Regardless, even if it makes hydrogen peroxide with water the main line of reason here is you drive the formation of the less soluble boric
acid by acidifying the enviorment, a solution of sodium perborate in water, acidified, will precipitate boric acid. The anologous reaction between
sodium borate and hydrochloric acid is covered elsewhere on this forum. So in the end you have a solution of hydrochloric acid, sodium chloride, and
hydrogen peroxide and a small amount of solvated boric acid, the majority of it precipitating.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I have an idea that, in this reaction (if it works - precipitation of perboric acid is a possibility particularly at low temperatures), some of the
nascent (initially monatonic) oxygen liberated may oxidize the Cl- ions present to gaseous chlorine or hypochlorite. Also, in commercial or technical
or reagent (non-analytical) grades of HCl there are present traces of Fe(II) and Fe(III) which impart a slight pale yellow-green color, and it is this
that would greatly catalyze the decomposition of the peroxide linkage in the perborate, in the same way as Fe catalyzes the decomposition of hydrogen
peroxide.
|
|
|
ChemPhile
Harmless

Posts: 9
Registered: 1-11-2004
Member Is Offline
Mood: No Mood
|
|
So, what's the structure of the sodium perborate, bromic acid?
I find its structure from chemfinder, but it seems not correct!
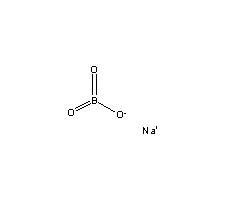
|
|
|
cyclonite4
Hazard to Others
  
Posts: 480
Registered: 16-11-2004
Location: is unknown
Member Is Offline
Mood: Amphoteric
|
|
I tried precpitating boric acid from a borax (sodium perborate) solution once, and had no luck. Two references I have here (Oxford dictionary of
Science, and McGraw Hill dictionary of chemistry) state that boric acid is soluble.
Would chilling the solution of boric acid or evaporating some of the water help precpitate the boric acid?
\"It is dangerous to be right, when your government is wrong.\" - Voltaire
|
|
|
TPL
Harmless

Posts: 12
Registered: 23-1-2004
Location: DK- Shit holed country!
Member Is Offline
Mood: Hmm..
|
|
Well, is there anything elso one can use Sodium Perborate for? I exspect it to be as pure as possible..
I've only found these thread's
http://www.sciencemadness.org/talk/viewthread.php?tid=262
http://www.sciencemadness.org/talk/viewthread.php?tid=3021
I want a lady on the street, and a wild bitch in the bed!
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
As for the structure of sodium perborate,
| Quote: | | SODIUM PERBORATE is a true inorganic peroxide containing a cyclic peroxide ring structure. |
From Solvay Chemicals I found some other interesting structures for it online too, depending on the state of the molecule, e.g., aqueous, hydrated,
anhydrous.
My main chemistry book states,
| Quote: | | In detergent formulations, it is no longer borax but sodium perborate, NaBO3, that is used. Once again, the simple formula does not show the true
structure of the ion, [B2(O2)2(OH)4]2- |
The cylcic nature of it agreed upone here as well.
As for the preparation of boric acid by acidification of a borate, I've never tried it, but it has been done sucessfully by a few members of this
board and they have posted on it. The solubility of boric acid according to Handbook of Chemistry and Physics is 1.95 g per 100 ml at 0 C and 39.1 g
per 100 ml at 100C. Sodium borate though appears to be significantly more soluble then the perborate, but decomposition may occur at somewhat
elevated temperatures.
As for other uses it appears to find some use in organic chemistry, something I found while trying to find a structure online, | Quote: | | Perborate but not percarbonate in acetic acid generates peracetic acid on standing and the peracetic acid oxidation of anilines is fast. The
oxidation with a fresh solution of perborate in acetic acid is smooth and second order but the specific oxidation rate increases with increasing
[perborate]0 or [boric acid]. Perborate on dissolution affords hydrogen peroxide and a borate; the latter assists the former in the oxidation. The
oxidation rates of anilines under identical conditions do not conform to any of the linear free energy relationships but the reaction rates of
molecular anilines do. Perborate oxidation proceeds via two reaction paths but the overall oxidation rates of molecular anilines conform to
structure–reactivity relationships; the transition states do not differ significantly. Analysis of the oxidation rates of perborate and percarbonate
reveals that while perborate oxidation is faster than percarbonate it is at least as selective as the latter. |
An abstract from J. Chem. Soc., Perkin Trans. 2, 2002, (12), 2011 - 2018
|
|
|