| Pages:
1
2 |
TheCopperMan
Harmless

Posts: 30
Registered: 4-5-2014
Member Is Offline
Mood: No Mood
|
|
Experimental: Alternative to Al/Hg - the Al/Ga
References
There has been plenty of talks about using gallium to activate Al, both on this forum and elsewhere. Search Google for "Al/Hg gallium sciencemadness"
and you will find many posts referencing the use of gallium. However, it seems there has been no complete writeups of this method.
So, after some experimentation, a general method has been found that produces a reaction VERY similar to an Al/Hg and in the same yields too. And
unlike an Al/Hg it can be scaled massively, because the amalgam itself is added gradually.
So far it has been tried on a propene which is notoriously hard to reduce, and gave the same yields as Al/Hg.
Experimental
Amalgam preparation
Many methods have been tried to prepare the amalgam. It has been tried making gallium salts, but they show very little reactivity or willingness to
attack the Al. It has been tried using plain Ga, and dissolving Al powder, foil, etc. into the plain Ga, and generally all methods took very much
time, required heating and other nonsense.
The easiest method found was this (takes 10 min tops):
Measure up 15g molten Ga in some disposable container. Now take a large sheet of Al-foil, keep it as straight as possible, and fold it like you were
folding a piece of paper very precisely, making sure it's completely flat before you do the next fold. Unlike paper, it can be folded more than 7
times (or was it 9, don't remember)  . You will be left with a thick 'brick' of Al.
Make a total of 12g worth of such bricks. Takes maybe 3 minutes. Now, put them into the container containing the Ga. Take a teaspoon (or etc) and
exert strong downward force on the bricks in the Ga container, whilst scraping them back and forth into the Ga. This way, the Al dissolves AMAZINGLY
quick. It takes maybe 5 minute tops until you have a uniform, silvery mass of amalgam. That's it, your amalgam is done. . You will be left with a thick 'brick' of Al.
Make a total of 12g worth of such bricks. Takes maybe 3 minutes. Now, put them into the container containing the Ga. Take a teaspoon (or etc) and
exert strong downward force on the bricks in the Ga container, whilst scraping them back and forth into the Ga. This way, the Al dissolves AMAZINGLY
quick. It takes maybe 5 minute tops until you have a uniform, silvery mass of amalgam. That's it, your amalgam is done.
Sidenote: It is strongly recommended to add maybe 5% tin (based on weight Ga) into the amalgam, to lower the MP of the amalgam. This makes it react
much better and more completely (instead of reacting just a bit then getting covered with sludge that prevents further access to the amalgam). It also
makes it a bit softer and easier to handle.
Reaction
Here, you can use the regular Al/Hg solvent ratios, unlike with the Al/Cu. Add all the ingredients together. Recommended to use 10g Al per 10g
substrate at least. No need to apply heat. Now, start adding the amalgam bit by bit, into the reaction flask. Use gloves, break off pieces of amalgam
with your hands, roll them into long sausages or whatever shape maximizes the surface area, then add it through an open joint (close joint after each
addition). Add it however fast or slow you want, depending on if you want a volcano type reaction or just a nice, slow reflux.
After finished adding, reflux reaction for a bit, whatever you want, then work up as usual.
Some notes
Because this reaction can be easily controlled by the addition rate of the amalgam, it has been thought that this reaction can easily be scaled up
VERY large, AND solvent volumes can probably be reduced, allowing to use much less size glassware for much larger batches of substrate.
|
|
|
Crypto
Hazard to Self
 
Posts: 50
Registered: 18-11-2013
Member Is Offline
Mood: No Mood
|
|
10g of aluminum per 10g of substrate, but how much amalgam? Let's say someone wants to run the reaction with 100 or even 200 grams of aluminum. A gram
of HgCl2 would be enough. How much Al/Ga? All the aluminum is suppose to be mixed with gallium?
[Edited on 29-6-2014 by Crypto]
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
Just for the record, I have used about 1/10 the mass of gallium as TheCopperMan has described and while I did not end up with liquid amalgam but
rather an aluminum sample that looked like it was suffering from "tin disease"  it
worked well. Similar to the results that TheCopperMan described, my experiments showed performance nearly identical to Al/Hg amalgam system. it
worked well. Similar to the results that TheCopperMan described, my experiments showed performance nearly identical to Al/Hg amalgam system.
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
TheCopperMan, thanks for your suggestion to use a bit of tin to reduce the MP. I will definitely keep that in mind for the next time.
|
|
|
Crypto
Hazard to Self
 
Posts: 50
Registered: 18-11-2013
Member Is Offline
Mood: No Mood
|
|
Any success recovering Ga? I have 10 grams of it just waiting to play with it. Would be nice to recycle it because it is quite expensive at my
sources. About 3000 dollars per kilogram.
|
|
|
TheCopperMan
Harmless

Posts: 30
Registered: 4-5-2014
Member Is Offline
Mood: No Mood
|
|
@Burner
Indeed, the amalgam is not liquid, but some mixture between liquid and solid.
@Crypto
It can be recycled, approx 95% is recovered per reaction. If one is less sloppy then more can be recovered. After reaction is finished, NaOH has been
added (dissolving all sludge) and reaction extracted, the gallium should be a liquid pool at the bottom. Use your imagination to get it out, then wash
it with some water, some NaOH and lastly some HCl or better yet H2SO4 to dissolve the aluminum oxide, and it's ready for another reduction.
10g Al per 10g substrate per 15g Ga is the ratio.
Also, 3000 per kilogram? On ebay it is sold for 340EUR per kilogram.
[Edited on 29-6-2014 by TheCopperMan]
|
|
|
pepsimax
Hazard to Self
 
Posts: 74
Registered: 30-7-2013
Member Is Offline
Mood: No Mood
|
|
Damn you copper man, I've only just got my shed clean from the copper experimentation.
I'd recommend gloves for making the alloy, the silver fingers last an eternity. I still have a very pretty mirrored beaker however! Try it in an old
one 
[Edited on 29-6-2014 by pepsimax]
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
My idea to use gallium came from this post - http://www.sciencemadness.org/talk/viewthread.php?tid=11687 and was surprised that it worked extremely well, equivalently to Al/Hg amalgam.
I have been reading voraciously since I joined here!  The archives have some
really great info. The archives have some
really great info.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Yes, Da, Si, Oui, Ja!!! Not to mention fantastic members.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
BTW, this is what I used to realize that it was possible to use much less gallium.
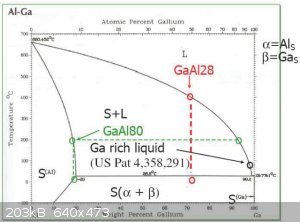
|
|
|
TheCopperMan
Harmless

Posts: 30
Registered: 4-5-2014
Member Is Offline
Mood: No Mood
|
|
Hm, it was tried to use less gallium at some points but this produced some kind of super-reactive dry powder. Seem to recall that it reacted instantly
once it touched the surface of the reaction and never really made it to the bottom...but this is very long ago and the memory is fuzzy
Generally though the less gallium the better, and the more surface area the better. Sometimes if you add too thick pieces of amalgam into the reaction
the piece will be covered in sludge and stops reacting unless you touch it with a stirr bar to expose the fresh amalgam underneath the surface and
restart reaction.
BTW Burner what was the substrate you reduced?
[Edited on 30-6-2014 by TheCopperMan]
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
I used Al shot (about the size of a BB [3-4mm dia]) rather than powdered Al that I suspect that others may have tried, and the Ga-reacted Al shot
provided a reactive material that was added through the reflux condenser a piece at a time, to control the speed of reaction.
I tested it against a number of unsaturated alkenes. I was pleasantly surprised that it worked so well. BTW, I am looking to test your Cu-mediated
approach sometime in the future to compare results with the Ga approach that I used.
|
|
|
pepsimax
Hazard to Self
 
Posts: 74
Registered: 30-7-2013
Member Is Offline
Mood: No Mood
|
|
I can't seem to get the gallium to react properly. An alloy made with a few bits of aluminum and gallium bubbles in water but in this ratio it does
nothing. I combined 750mg Ga with 500mg Al, it formed a dense liquid, some parts quite powdery. This was dropped in 15ml dH20, 20ml GAA, 15ml EtOH
with 500mg nitrostyrene in a 100ml conical flask. No reaction occurred after 15 mins. This was heated to 65c, again no reaction. Before writing it
off, I dropped 100mg copper chloride in there. Instantly it started bubbling so I began rapidly swirling the flask. The colour of the solution quickly
faded from yellow to clear then grey, dark grey and when the Al had gone it was a dark grey/red colour.
This was worked up in the usual way to give 380mg of PEA, which seems to be my best result from copper or Ga. Not sure if the Ga helped or what.
|
|
|
TheCopperMan
Harmless

Posts: 30
Registered: 4-5-2014
Member Is Offline
Mood: No Mood
|
|
I take it your Ga is very pure. Usually with recycled Ga combined with Al it will react pretty fast once it touches the liquid, however with fresh Ga
it would sometimes refuse to react at all. However if the heat is turned up to reflux for 2-3hrs, despite no bubbling or no seemingly activity, there
would be a color change and once worked up it still produced a decent yield. So even if it seems like nothing happening, there still might be
something going on. However this kind of behavior was as said only experienced with very pure, fresh Ga, once it was recycled it would become much
more reactive.
Adding copper was an interesting twist though. Not sure if the copper or Ga did the work, perhaps you could retry once with only Al and copper.
|
|
|
pepsimax
Hazard to Self
 
Posts: 74
Registered: 30-7-2013
Member Is Offline
Mood: No Mood
|
|
Ah interesting, thanks! The Ga was sold as 99.999% though I usually take that with a pinch of salt. I ran the above again and it proceeded in the same
way.
From the looks of things it seems that the gallium and copper help compliment each other. The "galluminium" sinks and breaks up so the Al is in the
solution at all times. The copper chloride attacks it preventing any sludge building up and slowing the reaction. Though this could be wishful
thinking!
Ga alone gave no product but I didn't try the 3h reflux, copper gave product but lots of strange side products which varied each time. The same
nitrostyrene gave a pure and good result with the combo. Run out of gallium now but it would be interesting to see if anyone else reported the same

|
|
|
TheCopperMan
Harmless

Posts: 30
Registered: 4-5-2014
Member Is Offline
Mood: No Mood
|
|
Generally Ga alone should give the same results you are seeing, assuming your amalgam actually shows a reaction once it touches the liquid. (even if
it doesnt a 2-3hrs reflux should do and give the same yields). To make the amalgam more reactive one can for example use recycled Ga which is a bit
more impure and allows easier access to the Al inside, or you can also use less Ga per Al. Like Burner who used 1/10 the amnt with Al shot.
As for the copper, it seems to be less clean than Ga indeed and side products were observed but they were always consistent and they seemed pretty
insignificant. Of course nothing like NMR or gcms is available...what were yields for copper?
[Edited on 22-7-2014 by TheCopperMan]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I think you should do some "scaling" experiments to see how little Ga you could use and write this up for ACS.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
pepsimax
Hazard to Self
 
Posts: 74
Registered: 30-7-2013
Member Is Offline
Mood: No Mood
|
|
Wanting to try a different substrate, bit bored of phenethylamine after 20 odd experiments with your procedures... Nitromethane is the only thing I
have to hand.
I must admit I'm uneasy about using the stuff, potential explosions are not something I'd look forward to. Been reading up and saw nitromethane and
copper chloride are incompatible but can't figure out why, anyone know? Rest of the procedure shouldn't cause problems, except extra care when basing
in case of residual nitro ?
|
|
|
pauldir
Harmless

Posts: 5
Registered: 16-8-2014
Member Is Offline
Mood: No Mood
|
|
some tests with the Al/Gu, this show me first a strong reaction when the Al/Gu comes in contact with water, this reaction stops some seconds later and
Al stay in the bottle. Some mixing will not help.
In acetic acid (80%) the reaction starts not (in pure dest. water starts immediately)
... we will see. Next time i try the preparation step by step from the first posting.
[Edited on 17-8-2014 by pauldir]
|
|
|
ChemicalCowboy84
Harmless

Posts: 4
Registered: 29-6-2014
Location: the great white north
Member Is Offline
Mood: great
|
|
What about using nitromethane instead of methylamine? Will this reaction still work any precaution need to be taken ?
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Yes absolutely. The addition of another exothermic reaction to an existing exothermic reaction (I think it reacts faster too? confusing..) definitely
makes it less predictable for an Al/Hg, so I would take similar precautions. Get a good feel for the reaction at small scale in a large flask and
scale it up slowly. Some people have had their reaction go from the flask to the roof using nitromethane in Al/Hg instead of methylamine.
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by pepsimax  | I can't seem to get the gallium to react properly. An alloy made with a few bits of aluminum and gallium bubbles in water but in this ratio it does
nothing. I combined 750mg Ga with 500mg Al, it formed a dense liquid, some parts quite powdery. This was dropped in 15ml dH20, 20ml GAA, 15ml EtOH
with 500mg nitrostyrene in a 100ml conical flask. No reaction occurred after 15 mins. This was heated to 65c, again no reaction. Before writing it
off, I dropped 100mg copper chloride in there. Instantly it started bubbling so I began rapidly swirling the flask. The colour of the solution quickly
faded from yellow to clear then grey, dark grey and when the Al had gone it was a dark grey/red colour.
This was worked up in the usual way to give 380mg of PEA, which seems to be my best result from copper or Ga. Not sure if the Ga helped or what.
|
If my math is correct, yield was 93.58 % amine ( calculated as free base)  . Well impressive! This anomaly should be investigated in my opinion. . Well impressive! This anomaly should be investigated in my opinion.
[Edited on 18-7-2016 by Mush]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Is an aluminum alkoxide a participating reagent in this reaction? Or are their formation too slow?
I know that aluminum triethoxide is a reducing agent for aldehydes and ketones, but not sure about what else.
[Edited on 19-7-2016 by Loptr]
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Loptr  | Is an aluminum alkoxide a participating reagent in this reaction?
[Edited on 19-7-2016 by Loptr] |
This is a dissolved metal reduction (internal electrolytic). Aluminum alkoxide can't form due to H2O persent.
https://en.wikipedia.org/wiki/Aluminium_amalgam
|
|
|
CRUSTY
Hazard to Others
  
Posts: 139
Registered: 5-6-2016
Location: Nearby
Member Is Offline
Mood: High-Order
|
|
I've heard the use of gallinstan (Ga/In/Sn) works pretty well as a lowered MP substitute for straight amalgam, as well as just tin, if one can't come
across and indium.
|
|
|
| Pages:
1
2 |