The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Distillation and steam distillation apparatus
Distillation and steam distillation apparatus:
Over the weekend I stayed at someone's cabin, and they had a percolator. has anyone ever used one for distillation? It seems to me if you popped
cinnamon in where the coffee grounds or tea would go, you could use it as a steam distillation rig, and get cinnamaldehyde where the brewed coffee
would be made. Just a thought. Has this been done before? Percolators seem like useful chemistry apparatus.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The term 'percolator' conjures up quite a few similar but different devices. Which type did you mean?
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
From what I can tell, http://en.wikipedia.org/wiki/Coffee_percolator
The one I saw had the coffee at the bottom, with a water bath beneath, which steamed it, going up a tube to the upper half, where it would condense.
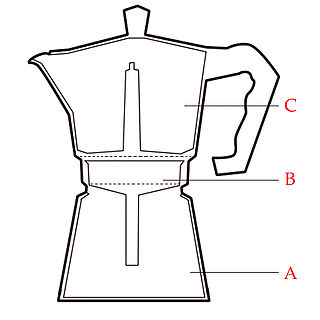
Like this one in shape.
[Edited on 5-26-2014 by The Volatile Chemist]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
That looks more like a Soxhlet extractor (with no siphon) than a steam distillation apparatus.
[Edited on 26-5-2014 by Cheddite Cheese]
|
|
|
DrAldehyde
Hazard to Self
 
Posts: 82
Registered: 12-1-2014
Member Is Offline
Mood: No Mood
|
|
Speaking from experience, that is known as a Cuban coffee pot in some circles
http://www.google.com/imgres?imgurl=http://www.espresso-mach...

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's exactly what I meant Dr.Ald. ! What's your opinion on how they'd wwork as a steam disillation unit?
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
If you would have used:
http://www.sciencemadness.org/talk/search.php?fid=5
you should have found:
http://www.sciencemadness.org/talk/viewthread.php?tid=5950
which should answer most if not all of your questions  The attached document
might be helpful as well The attached document
might be helpful as well  The end results from the effort applied The end results from the effort applied 
Attachment: SteamDistillation.pdf (792kB)
This file has been downloaded 798 times
Chemistry is our Covalent Bond
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Sorry, this is not a UTFSE situation. I guess you don't understand that we are talking about the usage of a COFFEE making device as a chemistry tool,
not just discussing steam distillation.
|
|
|
DrAldehyde
Hazard to Self
 
Posts: 82
Registered: 12-1-2014
Member Is Offline
Mood: No Mood
|
|
It wouldn't work for steam distillation. The unit boils water, which is forced up the tube as liquid. They make great espresso though.
|
|
|
Burner
Hazard to Others
  
Posts: 100
Registered: 28-3-2014
Member Is Offline
Mood: No Mood
|
|
^^^ Wouldn't cutting off the tube to a place above the water level solve that issue?
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Sorry, this is not a UTFSE situation. I guess you don't understand that we are talking about the usage of a COFFEE making device as a chemistry tool,
not just discussing steam distillation. |
Using Wikipedia should have found:
http://en.wikipedia.org/wiki/Steam_distillation
and
http://en.wikipedia.org/wiki/Percolation
and thus answered these queries  The extraction of cinnamon is discussed in the
attached document which was found freely online using Google The extraction of cinnamon is discussed in the
attached document which was found freely online using Google  The end results
from the effort applied The end results
from the effort applied 
Attachment: Practical_Flavoring_Extract_Maker.pdf (1.4MB)
This file has been downloaded 517 times
[Edited on 27-5-2014 by leu]
Chemistry is our Covalent Bond
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Volatile chemist:
I think that would work. I have one of those, they're very popular in Italy (where I lived for a few years), to make expresso coffee.
Once the water in the lower compartment boils, a mixture of steam and boiling water is forced up the tube and through the ground coffee and into the
upper compartment, extracting the drinkable coffee extract from the ground coffee.
While not perfect I think it could be used as a simple steam distillation apparatus. Only one way to find out...
@leu: the question was specifically about this percolator, not about steam distillation or cinnaldehyde in general.
[Edited on 27-5-2014 by blogfast25]
[Edited on 27-5-2014 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Percolating coffee pots use steam generation to carry a mix of hot water and steam to the top of the pot where the boiling water & condensate fall
back onto the coffee grounds and extract the soluble matter from the grounds.
That's not what you want from a steam generator - you want just steam. In fact I often use a "steam trap" following my steam generator (a pressure
cooker) just to remove condensate from the steam.
Is what you are proposing any different, in principal, from a regular percolating coffee pot?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | That's not what you want from a steam generator - you want just steam. In fact I often use a "steam trap" following my steam generator (a pressure
cooker) just to remove condensate from the steam.
|
In what way would some hot water affect the extraction though?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Magpie  | That's not what you want from a steam generator - you want just steam. In fact I often use a "steam trap" following my steam generator (a pressure
cooker) just to remove condensate from the steam.
|
In what way would some hot water affect the extraction though? |
Hot water would be great for extraction as in making coffee, or as the OP suggests, extracting other soluble material from some substrate like
cinnamon. I just don't see the point of trying to use such a device for "distillation." I suppose it could be used with some modification for steam
distillation. I usually prefer to do steam distillation by injecting dry steam, however, as it is more efficient.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Magpie  | Quote: Originally posted by blogfast25  | Quote: Originally posted by Magpie  | That's not what you want from a steam generator - you want just steam. In fact I often use a "steam trap" following my steam generator (a pressure
cooker) just to remove condensate from the steam.
|
In what way would some hot water affect the extraction though? |
Hot water would be great for extraction as in making coffee, or as the OP suggests, extracting other soluble material from some substrate like
cinnamon. I just don't see the point of trying to use such a device for "distillation." I suppose it could be used with some modification for steam
distillation. I usually prefer to do steam distillation by injecting dry steam, however, as it is more efficient. |
Right. I guess I misused the term steam distillation.
As a side note, percolators are different than standard coffee makers.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
There are subtle differences between using steam to extract something and hot water (even mixed with steam). There are about a dozen or more common
methods of extracting compounds from organic material: steam distillations, percolations, continuous extractions, soxhlets, supercritical extractions,
liquid solvent extractions, etc... Each has differences in what compounds it removes and what compounds stay behind, as does the solvent used (if
other than water/steam), the temperature, the time, etc.
For instance, you would not want to use hot water to decaffinate coffee, as it would remove all of the "coffee" flavor as well. but supercritical
extraction does a pretty good job of removing the caffeine without all of the flavors (note, I will prefer regular coffee). So depending on that
task at hand, each method may produce a different mixture of materials extracted. The trick to becoming a good scientist to to either research which
method is best or do the experiments needed to find it. In natural product extractions, often one method will bring a particular component out better
than others and with less other materials contaminating it.
|
|
|