| Pages:
1
2
3 |
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Pictures of Element collections
How about a thread for pictures of element collections?
I would like to see what everyone's collection look like. There aren't a lot of pictures that I've seen, and since it seems like a lot of people here
have one, why not show them? After all that's what they are for right?
I would start, but mine is so pathetically small so far... I'll post it after some of the more complete collections.
Here's a video of Mrhomescientist's awesome collection.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This is my collection of the elements: http://woelen.homescience.net/science/chem/compounds/index2....
I have most of them, just a few remain to be collected.
I now also have samples of Pt and a few better samples of other elements. These pictures will be added soon.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
My current element collection:

Whole thing. Note the lack of gases of any kind, save for some store-bought ampoules of Ne and Ar.

Li, Be, B, and C.

Ne, Na, Mg, Al, Si, and S.

Ar, K, Ca, Ti, and Cr. Mn was supposed to be in this picture, but I took it wrong.

Ni, Cu, Zn, and Ga.

Br, Sr, and Zr.
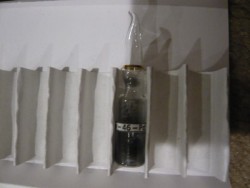
Pd.

In, Sb, and I.

Hf (newest addition!).

Pt, Au, and Hg. (shiiiiny....)

Lastly, Pb and Bi.
Some individual shots:

Nb and Mo. Can't fit these into ampoules just yet, and it took prolonged hammering to get Nb to its current state.

Lithium, finally in its own ampoule. Tried melting some of this stuff earlier today - no dice.

A comparison of my three alkali metals.
I'm not very good at photography, unfortunately - I bet kristofagyok could make even these look amazing. Oh well.
[Edited on 2-19-2014 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Looks a lot better than mine... I guess I could start collecting and keeping some of the gasses (H2, He, N2,O2
Cl2 and Ar), even though I can't amplule them. That would bump my collection to 19, which isn't bad.
[EDIT] I'll start with hydrogen, oxygen, and chlorine today because they will be the easiest. Nitrogen, I can just ask for a sample at a tire refill
place, maybe the same with argon, helium from a balloon...
[Edited on 19-2-2014 by Zyklonb]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Samples of colorless gases are not interesting at all. You could as well ampoule some air, nobody will ever notice the difference. For that reason, I
purchased the spectrum tubes which contain the colorless gases at low pressure and allow gas discharge spectra to be made. In that way you can
distinguish.
Some companies also sell simple ampoules with gasses at low pressure. These also can be distinguished from each other, by turning a few rounds of wire
around them and applying a high frequency high voltage on the wire. Due to capacitive effects, a small alternating current flows through the gas and
this causes color effects which are different for different gases.
http://www.smart-elements.com/?arg=detail&element=Ar&...
Unfortunately these nice samples are quite expensive.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I know they aren't interesting, which is why I haven't bothered to waist any containers with them. But I haven't really made any additions to my
collection recently, so I should start building it up some more... Chlorine at least has some color so I'll definitely do that today, I've made it a
bunch of times for experiments, but never to store it (except to test a container once). I know I'll get some spectrum tubes which contain the
colorless gases at some point, but I'm not there yet.
[Edited on 19-2-2014 by Zyklonb]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thanks for the bump on my collection! I've added a lot of new samples since that video. I only have 16 left to acquire! I've been meaning to make
another video showcasing each one individually but I'm never happy with the lighting. Tough to get rid of the glare on those glass vials. Someone
recommended a polarizing filter, which I'd like to try but I need to buy one.
As for the colorless gases, I agree they can be boring. With mine, I always try to have an interesting story behind them or at least an interesting
container. For neon and krypton, I have little bulbs for indicator lights and flashlights. Argon was collected by putting an incandescent lightbulb in
a bag, filling it with water, and breaking the bulb inside the bag inside a larger bucket of water. I could then carefully collect the gas in the bag.
(Lightbulbs are a surprisingly rich source of elements!) Nitrogen is in a standard vial, but I filled it by dipping it in liquid nitrogen and allowing
it to boil off before capping, to displace the air. I tell people that it looks like nothing is in there, but you'll just have to believe me when I
relate the story 
EC1: Your K and Na look like really nice blobs. Did you make them yourself?
[Edited on 2-19-2014 by MrHomeScientist]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
You're welcome, Are you sure that incandescent light bulbs are pure argon? I thought they are a mixture of nitrogen and argon under reduced pressure.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I'd read that light bulb filler gas is either nitrogen or argon at low pressure, but I've never been able to confirm that with the bulb manufacturers.
I suppose I don't really know for sure what gas it is. I wonder if the wire wrapping technique woelen mentioned would work on a light bulb? Then you
could distinguish by the color of the glow. Or see if lithium blackens under bulb gas, indicating reaction with nitrogen.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Or calcium would also work, as it reacts with nitrogen too.
on wiki it says The bulb is filled with an inert gas such as argon (93%) and nitrogen (7%).
[Edited on 19-2-2014 by Zyklonb]
|
|
|
thebean
Hazard to Others
  
Posts: 116
Registered: 26-9-2013
Location: Minnesota
Member Is Offline
Mood: Deprotonated
|
|
@woelen: I looked at your element collection and saw that your chromium was produced through electrolysis. Out of curiosity do you have a procedure
that you could share? I couldn't seem to find anything on it here on the forum and Google yielded very little.
@MrHomeScientist: Light bulb gases tend to be either xenon or argon and sometimes nitrogen, when you get a halogen bulb you get very small quantities
of iodine or bromine added.
"You need a little bit of insanity to do great things."
-Henry Rollins
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Ok, so here is my collection:
  
Lithium, Carbon, Magnesium
  
Aluminum, Iron, Copper
  
Zinc, Iodine, Gold, Lead.
Can somebody please delete the last image, it replaced my lead, and I can't delete it, it just comes back.
[Edited on 20-2-2014 by Zyklonb]

[Edited on 20-2-2014 by Zyklonb]
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Just absolutely amazing to see all those elements, I love looking at these collections! Keep up the wonderful work everyone! 
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I also decided to post a picture of chlorine, It's not a very good sample, it still has water in it, may not be 100% pure, oh well. Also, I forgot
sulfur:
 
[EDIT] I tried to post it in my other reply, but I guess I had already posted to many pictures.
If you're wondering about cadmium, which I have isolated most recently, I had a use for it (which the reason I made it) and it's already used up. I
should have taken a picture
[Edited on 20-2-2014 by Zyklonb]
[Edited on 20-2-2014 by Zyklonb]
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
I must say Woelen's collection is chemist porn :p I discovered what many elements you never hear about looked like! By the way your sample of chlorine
looks great anyways zyklonb, the yellowish color is quite as intense as it can be. Of course when you'll have time making a sample without water would
be a good idea however.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Sodium: Nope, bought. Of course, it was in one of those hexagonal-prism foil packs from United Nuclear, so I pressed a few slices into a wiry sort of
shape, slitted them down through the ampoule neck until it was full, and then melted them inside the ampoule under kerosene.
Potassium: Same thing, except this was homemade! Took a couple tries, though - my ampoules' necks kept breaking due to increasingly ridiculous
circumstances. This was sealed under mineral oil.
The problem with sealing ampoules full of hydrocarbon solvents is twofold and annoying. First, if the flame touches any residue of solvent (say, on
the walls, even) it leaves a black carbon residue that makes the ampoule walls brittle and significantly harder to seal (this is evident in my
ampoules of calcium, potassium and lithium). Second, it occasionally catches on fire if it's particularly volatile - though it seems to be easy to
blow out.
Looking at my ampoules of strontium and calcium, and then at my ampoules of sodium and potassium, it's easy to see which group lithium deludes itself
into acting like. 
16 left! Which are they?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Hi Here are some pictures of my favourite elements in my collection.
    
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Cesium and Bromine look amazing! Is that white phosphorus, to the right of the red?
I think that iodine is my favorite out of mine, the crystals look so much better in real life. Lithium is my most irreplaceable, although the gold is
worth more.
[Edited on 20-2-2014 by Zyklonb]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Lots of really nice samples. Great thread idea! I'll have to take pictures of all of mine and make a blog post about them. Might be too many photos to
post them all here 
Zylkonb: I've never seen aluminum like that. It reminds me of the "sponge" of hairlike crystals I get from doing the silver tree demo
(silver nitrate + copper wire). I also laughed at the big CAUTION label on the copper wire.
elementcollector1: Sounds like a pretty hairy process to get those things into ampoules! I remember that Zan Divine had a thread
about ampouling alkali metals that had some pretty creative solutions. For my collection, the elements I have yet to collect are K, Ca, As, Rb, Nb,
Pd, Sb, La, Ce, Nd, Pm, Ho, Tm, Lu, Hf, and Ir. Technically I'm still missing F and Am, but I have representative compounds for each and that's
probably the best I'll be able to do (Teflon for F and an AmO2 smoke detector button). I also have made K but haven't been able to refine
it to presentation quality worthy of the shelf quite yet. I use uranium ore to represent Po - Pa, because of the decay chain of U, but I do still need
U and Th metals. Finally, I just realized Pm is probably uncollectable due to its radioactivity. So depending on how you look at it, I might have a
couple more than 16 left 
nezza: That cesium is gorgeous. What else is in the vial with your bromine? Something to absorb vapors?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
In case anyone likes, for the cost of postage, I don't mind donating a couple pieces of Sb to those seeking samples. The Sb I have can be seen in this
thread(you may need to scroll down)
http://www.sciencemadness.org/talk/viewthread.php?tid=26378
Likewise for representative F compounds I don't mind donating samples of calcium fluoride, as I have quite a bit.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I could also give a some samples (5-10g) freely of the Al from the picture, it's pyro grade bright flake Al ~27 micron treated with anti dust powder.
(From Skylighter.)
I would love some Antimony, Mailinmypocket. I would rather trade something than pay for postage, it just seems a lot easier.
[EDIT] CAUTION contains, Zinc May cause blindness, WARNING Iron filings, May be attracted to magnetic source, CAUTION Copper wire, May cause
electrical shock if connected to HV source.  ...It was from my old chemistry
set that I got when I was like 5 or 6. ...It was from my old chemistry
set that I got when I was like 5 or 6.
I got some iron that was melted in a thermite. Here it is:
[Edited on 20-2-2014 by Zyklonb]

|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Zyklonb. Yes that is white phosphorus next to the red.
MrHomeScientist. That is just some tissue to pad the bottom of the universal.
One more picture, not an element, but recently I have had a go at making some NaK and ampouling it under argon. This is my first attempt. There is
some oil in the tube as well which spoils the appearance a bit.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Cool... I'm too scared to make NaK, at least with the limited apparatus and equipment that I have.
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Mailinmypocket I would love to get a small sample of Sb if you are offering. Its hard to find any amount in small quantity online for a reasonable
price, so I've been holding off on buying it.
In the spirit of the thread here is a picture of my element collection:

Click here for a larger view.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Just a note for those looking for antimony samples, since I have received a few requests. If you would like some please PM me as I don't always
remember to check back on threads and may forget about it.
|
|
|
| Pages:
1
2
3 |