| Pages:
1
2
3 |
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Distilled water density
Hi all,
I keep noticing that when I weigh samples of the distilled water that I use (mostly from Target but sometimes from McMaster-Carr, gives similar
results), I get slightly less weight per volume than I would expect. Today I did an experiment where I took three different 25mL volumetric flasks,
weighed each one three times empty, then three times when full of water (bottom of the meniscus on the cal line), and averaged the readings for each
flask and took the difference. I get the following densities for the water in the three flasks: 0.9943, 0.9935, and 0.9930 g/mL.
These are class A volumetric flasks calibrated for 20 C. The room was 19 C and the water was 19.7 C. My analytical balance shows a maximum possible
error of 0.016% on a 150g cal weight (including the tolerance of the weight).
Pure water should be 0.998 g/mL. The difference between this and the result I get, while small, is several times larger than can be accounted for due
to my sources of error.
Any idea what is up? Would there be enough impurities in distilled water to cause this much density change?
Thanks!
Sean
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
dissolved CO2, N2 & O2
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thanks, chemrox. Based on a paper by NIST (Effect of Dissolved Air on the Density and Refractive Index of Water by Harvey, Kaplan, and Burnett), at 20
C this should only make 0.002 g/mL difference, which means an expected density of 0.996 - still 0.002 away which is still more than the sum of the
effects of air buoyancy and analytical balance error, although it is getting much closer so maybe I need to be more careful with my error budget.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
OOPS, no, I just made a big mistake. That paper says that the dissolved gasses should only make 0.002 mg/mL difference - totally in the noise, so no,
the dissolved gasses do not explain what I am seeing.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Don't forget the influence of pressure, temp. and R/H?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Seems to me the error is in line with what to expect from using volumetric flasks, as opposed to picnometers. Your average is 0.9936 or about 0.0044
off the listed value of 0.998. Times that by 25 and you get 0.11 g or about 0.1 ml.
I don't think you can expect much better even with Class A volumetric flasks.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
hissingnoise: I have accounted for temperature in that I used the 20 C value for water density and my volumetric flasks were being used very close to
their rated temp (19 C versus 20 C - should make a difference far less than I am seeing). Air buoyancy effect is not nearly large enough to cause this
amount of error, either, so I don't see how pressure and RH would matter.
blogfast25: These volumentric flasks are rated +/- 0.03 mL. Also, the error is consistently the same sign across three flasks and the variation from
flask to flask is small-ish compared to the overall discrepancy. It is clear that these volumetric flasks were individually calibrated because when
you put them next to each other, they are all the same model number but their calibration marks are not at the same height.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I ordered a bottle of ultrapure HPLC-quality water and when it arrives I will try this again with that water.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Have you considered the calibration of your balance? You say it reads well with a 150g calibration weight but is it a single point calibration or a
span calibration? Have you checked what readings it gives with other standardised masses? Particularly in the region of what the volumetric and 25 mL
water weighs (50-60 g?). Balances for cGMP use where I work undergo a seven-point calibration to account for such non-linearity.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Ockham's Razor — User Error
From the descriptions, it sounds to me like your balance isn't properly leveled. Close one eye and look at the bubble from directly above to avoid
parallax error when adjusting the leveling feet. The bubble must be dead center under the printed circle, or your weight values will read
consistently low. In the diagram below, you want σ = W, θ = 0.
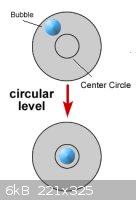 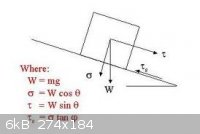
[Edited on 12.2.14 by bfesser]
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
there is also the fact that he can never know exactly when the flask is filled to the line (assuming he is human)
all above information is intellectual property of Pyro.  |
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thanks for all the replies. I do agree that the most likely reason is some kind of user error or problem with the instruments. However, I do not yet
see how.
Balance calibration: Unfortunately, the only cal weights I have are 150g, 500mg, and 100mg. The 500mg and 100mg are expensive class 1 weights and the
150g is a cheap class M1, but still supposed to be within 7.5mg. Here is what I get when I weight each one of these weights in sequence several times:
149.9832g, 0.4999g, 0.0996g, 149.9821g, 0.4998g, 0.0999g, 149.9822g, 0.5003g, 0.1000g
Balance levelling: the bubble is within the inner circle, although the place where I have the balance I cannot get my head directly above it because
of cabinets above. However, to cause 0.004 scale error would require 5 degrees of tilt which would be clearly noticeable.
Filling the volumetric flasks: I can very easily tell the difference between being above or below the line to within one eyedropper drop with a
dropper which is about 1/20 mL per drop, which would be half of the observed error. It would seem strange that I get it consistently wrong in the same
direction, but maybe.
|
|
|
phlogiston
International Hazard
    
Posts: 1381
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
To conclusively exclude sources of variation as the reason for your result you should repeat your experiment, but this time use the same flask for all
measurements and repeat the entire procedure (each and every step of it from begining to end (filing etc)) for each measurement.
Then, calculate the average and standard deviation of your measurements and show that the difference is really statistically significant.
Once you have unequivocally shown that there is a significant difference in density, the discussion will shift to the WHY question.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sbreheny  | I do agree that the most likely reason is some kind of user error or problem with the instruments.
. . .
Balance levelling: the bubble is within the inner circle, although the place where I have the balance I cannot get my head directly above it because
of cabinets above. However, to cause 0.004 scale error would require 5 degrees of tilt which would be clearly noticeable.
Filling the volumetric flasks: I can very easily tell the difference between being above or below the line to within one eyedropper drop with a
dropper which is about 1/20 mL per drop, which would be half of the observed error. It would seem strange that I get it consistently wrong in the same
direction, but maybe. |
I'll assume that you neglected to account for friction in your calculation of
5° tilt. If you can't get your eye directly above the bulb, use a mirror.
Another simple explanation; are you correctly reading the maniscus? Reading from the sides or from a high angle could account for your consistently
low values.
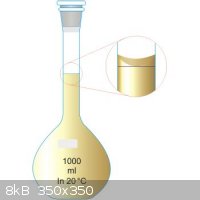 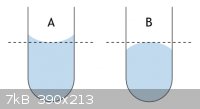 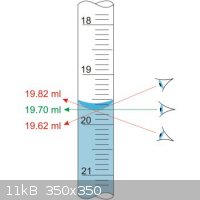
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Try using a pycnometer:
https://www.google.co.uk/search?q=pycnometer&rlz=1T4DSGP...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
An interesting distilled water-related situation I came across: I recently judged a science fair for middle and high schoolers, and one of the
projects involved measuring condutivity of different drinks to find the relative amount of electrolytes they contained. In the experiment she claimed
she got some conductivity from distilled water. If it was pure water, of course, it shouldn't conduct at all.
I haven't done this experiment myself, and it was a middle-schooler's project, so this is all hardly conclusive. Still, another interesting indication
that "pure" distilled water from the supermarket might not be quite so pure.
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
In which case, your density measurement is accurate to one significant figure of precision.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I'm not sure I understand what you mean. 0.03 is much more than one sig figure out of 25mL, and certainly my balance is accurate to much more than one
sig figure out of 25g, so the end result should have more than one sig figure of accuracy.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | I'll assume that you neglected to account for friction in your calculation of 5° tilt. If you can't get your eye directly above the bulb, use
a mirror.
Another simple explanation; are you correctly reading the maniscus? Reading from the sides or from a high angle could account for your consistently
low values.
|
Regarding the meniscus, I am reading it at eye level by kneeling down, leaving the flask on the table, and aligning my eye with the calibration ring
so that the entire ring appears as a line and then lining-up the very bottom of the meniscus with the line.
Regarding friction: are you referring to friction caused by the lateral component of gravity placing a sideways force on the bearings of the weigh
platform? If so, then yes, I neglected that, but I think that should be a minimal effect because of the way the balance works. I believe that it
applies a constant dithering motion (microscopically - you can hear but not see it) so that dynamic friction cancels out and static friction cannot
occur because the platform is never truly stationary.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | An interesting distilled water-related situation I came across: I recently judged a science fair for middle and high schoolers, and one of the
projects involved measuring condutivity of different drinks to find the relative amount of electrolytes they contained. In the experiment she claimed
she got some conductivity from distilled water. If it was pure water, of course, it shouldn't conduct at all.
I haven't done this experiment myself, and it was a middle-schooler's project, so this is all hardly conclusive. Still, another interesting indication
that "pure" distilled water from the supermarket might not be quite so pure. |
Pure water still has some conductivity due to the equilibrium dissociation of water into OH and H3O ions. Also, the dissolved CO2 will contribute to
conductivity. Finally, and I think this is the greatest source of error, you cannot use a normal multimeter to measure water resistance because the DC
voltage used will begin to hydrolyze the water and cause an artificially-low resistance reading due to the extra ions produced. I think that you can
get a fairly good measurement by using an AC resistance meter. The electrode material may be important, too.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Doesn't that require a liquid of known density for comparison?
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Maybe another way to look at this is that 0.03mL is 30mg mass error. Out of 25mL, that is only about 1mg per mL of water. I am seeing an error of
about 3.5 to 4mg per mL error.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes. It determines relative density of a liquid, usually relative to distilled water at 20 C.
So you weigh the flask empty, then filled with distilled water, determine the weight of the water, divide that number by itself (so you get 1.0000).
Then multiply this by the tabled value for water density, and presto... problem solved! 
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>sbreheny</strong>, out of curiosity, are you stoppering the volumetric flask? Also, are you handling it with your bare (oily)
hands?
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | | <strong>sbreheny</strong>, out of curiosity, are you stoppering the volumetric flask? Also, are you handling it with your bare (oily)
hands? |
Yes, I am stoppering them and no, I was handling the flasks with nitrile gloves. Also, I cleaned the flasks with acetone before using them and made
sure they were completely dry before adding water.
|
|
|
| Pages:
1
2
3 |