Popov
Harmless

Posts: 3
Registered: 15-9-2013
Member Is Offline
Mood: No Mood
|
|
Reduction with Zn/HCOOH/PdCl2
Hello everybody!
I found a nice paper about the reduction of alkens with Zn/HCOOH/PdCl2.
B. Masesane, P. G. Steel, Bull. Chem. Soc. Ethiop. 2005, 19(1), 149-152.
| Quote: |
Abstract Catalytic transfer hydrogenation using palladium(II) chloride, zinc powder and various organic acids proved effective for the reduction
of a variety of alkenes at ambient temperature and atmospheric pressure. The method was found to be convenient, economical and uses a stable
nonpyrophoric catalyst.
|
Zn/HCOOH reduces nitrogroups and oximes to amines in high yields. Do you think Is it possible to reduce nitropropenes with Zn/HCOOH/PdCl2 direct to
phenylethylamines?
Regards,
Popov
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Popov  | I found a nice paper about the reduction of alkens with Zn/HCOOH/PdCl2.
B. Masesane, P. G. Steel, Bull. Chem. Soc. Ethiop. 2005, 19(1), 149-152. |
It does not make much sense to use zinc with formic acid when CTH hydrogenations work well with any simple formate salt (HCOOK, HCOONH4 or
HCOOH/Et3N are most commonly employed). The rest of the article, assuming it describes using Zn with other acids that themselves aren't
able to undergo the beta-hydride elimination, makes much more sense.
| Quote: | | Zn/HCOOH reduces nitrogroups and oximes to amines in high yields. Do you think Is it possible to reduce nitropropenes with Zn/HCOOH/PdCl2 direct to
phenylethylamines? |
What role would PdCl2 have here? Presumably, the hydrogenations of "nitropropenes" over palladium catalysts stop at the ketoxime stage exactly because
the electron poor double bond can't be reduced as fast as the nitro group. So I can't see what good could PdCl2 do in this case where Zn
would be used. See the thread Pd/C H2-gas reduction of ß-nitrostyrenes for more info.
In order to make the reduction go further than the ketoxime stage, you would want to add NiCl2 or some other nickel salt instead of
PdCl2, but then this is already a known system.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Popov
Harmless

Posts: 3
Registered: 15-9-2013
Member Is Offline
Mood: No Mood
|
|
Thanks for your reply!
Zn/HCOOH/PdCl2 should reduce the double bond in nitropropenes to yield nitropropanes. Nitropropane should be further reduced to the amine by
Zn/HCOOH.
The paper states that cinnamic acid is reduced to 3-phenylpropanoic acid in 84% yield and eugenol is reduced to 2-methoxy-4-propylphenol in 98% yield.
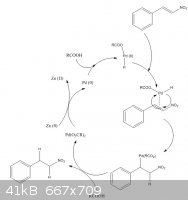
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
that journal is probably on the "axis of shtoopid", alongside indian and chinese journals.
Bullshit Chemistry Society of Ethiopia?
first thing that comes to mind here is the reduction of PdCl2 to insoluble Pd by Zn/HCOOH.
i highly doubt the validity of the procedure.
[Edited on 15-9-2013 by stoichiometric_steve]
|
|
|
Popov
Harmless

Posts: 3
Registered: 15-9-2013
Member Is Offline
Mood: No Mood
|
|
Maybe I will give it a try....when I manage to borrow some palladium chloride or palladium acetate at work. 
The paper is free available:
PALLADIUM-CATALYSED TRANSFER HYDROGENATION OF ALKENES IN THE PRESENCE OF ZINC POWDER AND VARIOUS ORGANIC ACIDS
| Quote: |
Typical procedure. To a stirred solution of alkene (1,4 mmol) in acetonitrile (20 ml) at room temperature was added PdCl2 (25 mg,
0,14 mmol), zinc powder (275 mg, 4,2 mmol) and acid (4,2 mmol). The reaction mixture was stirred for 16 h and then filtered. [...]
|
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You are being unduly offensive. The journal is just fine. I would not mind publishing there myself. Sounds so exotic that it would be kind of an
honour to have an article there.
The authors are not the usual Indian/Iranian suspects either. The first author is from Botswana. I did not even knew they had organic chemists, let
alone even chemist who publish in Org. Lett. and such (they even have a chemistry department at the University of Botswana!). I can only wish
them luck.
And the article is just fine as it is for what it is. The method is unfortunately relatively useless as such, given that it uses 10 mol% PdCl2 which
is an insanely huge amount for a simple hydrogenation, but they had some interesting results worth reporting. Trying out just three substrates, and
not evaluating the catalyst loading effect and the choice of solvent, is a bit of a downer, but we need journals where to publish short articles for
studies one does not have the time to get too involved with.
Quote: Originally posted by Popov  | | Zn/HCOOH/PdCl2 should reduce the double bond in nitropropenes to yield nitropropanes. Nitropropane should be further reduced to the amine by
Zn/HCOOH. |
That's whishfull thinking, but it does not work that way. That is no ordinary double bond. There is a nitro group there. Read the thread I refereed
you to in the previous post. The corresponding nitroalkanes are not intermediates in the hydrogenation of nitrostyrenes. There is a reason why
people use a two step reduction by first reducing the double bond with NaBH4 before they go on reducing the nitro group by whatever means.
Direct hydrogenation is problematic due to the ketoxime intermediate.
| Quote: | | The paper states that cinnamic acid is reduced to 3-phenylpropanoic acid in 84% yield and eugenol is reduced to 2-methoxy-4-propylphenol in 98% yield.
|
Cinnamic acid is not comparable and eugenol is something completely different.
Yet, I encourage you to do an experiment and try it out. There is nothing but a good old experimental verification.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Is there any difference between using formic acid and acetic acid here?
Does anyone know?
|
|
|