| Pages:
1
2
3 |
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Hexanitrobenzene
Would be pretty strong wouldn't it? One would think so. But hard to prepare. Couldn't be done by nitration of benzene.
But if one trinitrated benzene to 1,3,5-trinitrobenzene, then reduced all three nitro groups to amines, yielding 1,3,5-triaminobenzene, one should
then be able to trinitrate this to 1,3,5-trinitro-2,4,6-triaminobenzene. This would then have to be re-oxidized to 1,2,3,4,5,6-hexanitrobenzene.
That's a lot of work. But there might be another way.
Acetylenes trimerize to benzenes in the presence of Ni+2 catalysts. Some acetylenes with electron-withdrawing groups don't even need catalysts at all.
This reaction is not a minor one that has crappy yields, no no. These compounds want to trimerize.
Extremely sterically crowded benzenes have been prepared this way, ones that have yet to be prepared by any other method. Things like
hexa-isopropylbenzene, and 1,2,3-trifluoro-4,5,6-tri-isobutylbenzene, the first time in history that three isobutyl functions had ever been placed
next to each other on an aromatic ring.
Well, trimerization of 1,2-dinitroacetylene would yield 1,2,3,4,5,6-hexanitrobenzene. Hell, by all rights it should do it without a catalyst, but in
reality that kind of statement can't be made without some experimentation, so I won't make it. 
Dinitroacetylene should be VERY explosive, too, no? I would think that the aromatic compound would be much more stable than the acetylene when it
comes to shock at least.
My main question that I would like input on, is how would one go about preparing 1,2-dinitroacetylene? This very simple compound seems to be quite
difficult to prepare in any reasonable number of steps, and it is starting to frustrate me.
I got interested in this possibility after I heard of the reaction of 1,1,1-trichloroethane reacting with aluminum to form chloroacetylene. 'Hmm' I
said to myself at the time.
I can think of a few different pathways to 1,2-dinitroacetylene, but they all suck. What idea do you have? I am interested in hearing them, as well as
alternative ideas for the preparation of hexanitrobenzene, or any physical properties information on it, as well as explosive properties on it.
Whatever you can provide or take a wild stab at, I'd like to hear it.
PrimoPyro
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I was actually planning on giving 1,2,3,4,5,6-hexanitrobenzene synthesis a try sometime. There's a few ways it might be done without using benzene.
Nitration of 1-aminobenzene in mixed acid - I'm not sure if the nitration will go all the way to 1,2,3,4,5-pentanitro-6-aminobenzene. But if it did,
the 1,2,3,4,5-pentanitro-6-aminobenzene would then be oxidized with Caro's acid to yield 1,2,3,4,5,6-hexanitrobenzene.
Nitration of 1,4-diaminobenzene in mixed acid. I'm fairly confident the nitration will go all the way to 2,3,5,6-tetranitro-1,4-diaminobenzene.
1,4-diaminobenzene might be prepared by oxidizing xylene to its dicarboxylic acid, then reacting with urea, then reacting with calcium hypochlorite.
The 2,3,5,6-tetranitro-1,4-diaminobenzene would then be oxidized to 1,2,3,4,5,6-hexanitrobenzene with Caro's acid.
It is probably feasible to prepare 1,3,5-trinitrobenzene by oxidizing 2,4,6-trinitrotoluene's methyl group to a carboxyl group, then decarboxylating.
I've heard mentions of the decarboxylation proceeding easily - it supposedly can be done by boiling an aqueous solution of the 2,4,6-trinitrobenzoic
acid.
I weep at the sight of flaming acetic anhydride.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
As far as I'm aware, dinitroacetylene hasn't ever been isolated. It would make one hell of a (probably primary) explosive though...
I would bet a lot of money on the fact that it couldn't be done by direct nitration of acetylene though! A carbon-carbon triple bond, and
electrophilic nitronium ions... I can't think of any feasable ways to do it.
I know that HNB can be made from TATB, but I forget how exactly.
Aminopentanitrobenzene can be made without too much difficulty:
toluene -nitration-> TNT -oxidation, decarboxylation-> TNB -reduction-> 1-amino-3,5-dinitrobenzene -nitration-> APNB.
The -NH2 on the ring directs further -NO2's ortho/para in relation to it, and also activates the ring to electrophilic attack, thus making further
nitration MUCH easier than trying to nitrate sym-TNB.
And from APNB you're only some NaN3 away from CL-18, aminonitrobenzodifuroxan.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Madscientist, are you sure about Caro's acid doing the -NH2 to -NO2 conversion on aminobenzenes?
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
There is something that really interested me that PHILOU Zrealone mentioned in the trinitrotoluene preparation thread.
He said that nitration of an aminobenzene with sulfuric-free nitric acid will result in the nitration of the amine group to NHNO2; and then
the nitro group supposedly shifts onto the benzene ring, reforming the amine group. Has anyone heard about this?
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
It seems that PHILOU Zrealone didn't post that in the trinitrotoluene preparation thread. I know I saw it somewhere though...
Here's a quote from him, which is what caused me to think that oxidation with Caro's acid of an amine group will probably yield a nitro group.
| Quote: | How do you think it is possible to make hexanitrobenzene?
NH2-C6(NO2)4-CH3 -HNO3/H2SO4/H2O2(all 100%)-> C6(NO2)6 + CO2 + H2O |
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Triple post :D
This is something that PHILOU Zrealone posted in the trinitromelamine thread:
| Quote: | NH2-C6H5 + HNO3 (conc) --> O2N-NH-C6H5 + oxydation products (quinones)
As a mather of facts:
O2N-NH-C6H5 -rearranges-> NH2-C6H5-NO2 (para)
This proves an vicinal to aromatic ring nitramine is unstable! |
I weep at the sight of flaming acetic anhydride.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Ah, thanks for the quote. I looked in the TNT thread and couldn't find it! Although I did see the use of Caro's acid for -NH2 -> -NO2.
Damn, if it was meta then the C6H5-NH-NO2 --> O2N-C6H4-NH2 would be very useful for getting more nitrated aromatics, but since the product is para
it's of little use, since nitration of aminobenzene will get you p/o ANB anyway 
|
|
|
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Replies:
Madscientist:
| Quote: | | Nitration of 1-aminobenzene in mixed acid - I'm not sure if the nitration will go all the way to 1,2,3,4,5-pentanitro-6-aminobenzene. But if it did,
the 1,2,3,4,5-pentanitro-6-aminobenzene would then be oxidized with Caro's acid to yield 1,2,3,4,5,6-hexanitrobenzene. |
This will not work all the way to the fully nitrated benzene. At best it will stop at 2,4,6-trinitroaniline. (aniline is aminobenzene) But thats ok
too. I just wanted to point out that it will indeed not fully nitrate.
| Quote: | | Nitration of 1,4-diaminobenzene in mixed acid. |
Now this, on the other hand, is a great idea. This *should* be possible to fully nitrate.
| Quote: | | 1,4-diaminobenzene might be prepared by oxidizing xylene to its dicarboxylic acid, then reacting with urea, then reacting with calcium hypochlorite.
|
Won't work. Even if it did, it wouldnt work well. Firstly, terphthalic acid (p-benzenedicarboxylic acid) is cheap and available as is, or can be made
via cheaper methods, and also, Hoffmann Degradation does not work well at all on multiple functions on a single molecule. Most organic reactions work
horribly if multiple groups are worked on at the same time intramolecularly. This would be an example of such.
| Quote: | | It is probably feasible to prepare 1,3,5-trinitrobenzene by oxidizing 2,4,6-trinitrotoluene's methyl group to a carboxyl group, then decarboxylating.
I've heard mentions of the decarboxylation proceeding easily - it supposedly can be done by boiling an aqueous solution of the 2,4,6-trinitrobenzoic
acid. |
Thats a lot of dangerous work to get somewhere you can get to more easily from other methods. Would you want to subject TNT to KMnO4, H2SO4, and then
dry distill it with Ca(OH)2? I wouldn't.
Nick F:
| Quote: | | As far as I'm aware, dinitroacetylene hasn't ever been isolated. It would make one hell of a (probably primary) explosive though...
|
Damn damn damn, lol. I was afraid someone would say that (regarding its isolation). dinitroacetylene should be more powerful than HNB, gram for gram.
Keep that in mind. It was just a novel idea, that really should never be attempted. But HNB needs to be made, so someone can say they pimped
themselves some HNB just for the hell of it. [laugh]
Also, why go through all the trouble of ripping off that methyl of TNT when you can just trnitrate benzene? I understand that some of you cling to
your OTC ways, but when making research explosives, isn't it ok to cheat and assume you can get some benzene? 
Anyways, dont think Im dissatisfied, Im far from that.
I think the best method would be to mononitrate aniline with KNO3 (NaNO3 is not a reasonable substitute here, the K+ ion is crucial) to get
p-mononitroaniline, and then reduce this to p-diaminobenzene with Fe/HCl which is easy, high yielding, and cheap as hell.
Then nitrate the shit out of it with NaNO3 or LiNO3 or HNO3, but not KNO3 (yields will be lower) to get 1,4-diamino-2,3,5,6-tetranitrobenzene. This
could then be oxidized by several oxidizing agents to HNB.
PrimoPyro
|
|
|
obscura
Harmless

Posts: 1
Registered: 25-10-2002
Location: under hydrazin atmosphere
Member Is Offline
Mood: No Mood
|
|
HNB
You should stop the fucking post about
HNCHH ! 
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
TNB from benzene isn't easy (unless you do a Friedel-Crafts to make toluene!), it's much more economical to oxidise and decarboxylate TNT. That's the
way it's done. Otherwise, you get to m-DNB and then you have to use loads of oleum and 100% HNO3 and reflux it for hours.
TNBenzoic acid decarboxylates very easily, as madscientist said it can be done in boiling water with a bit of NaOH. I suppose since -COOH directs -NO2
groups to the meta position, and TNBenzoic acid has -NO2 o/p to the -COOH, this makes it less stable and more prone to decarboxylation than
3,5-dinitrobenzoic acid. I don't really know why, I haven't been taught about directing effects on aromatics and haven't got round to reading about it
yet.
[QUOTE]I think the best method would be to mononitrate aniline with KNO3 (NaNO3 is not a reasonable substitute here, the K+ ion is crucial) to get
p-mononitroaniline, and then reduce this to p-diaminobenzene with Fe/HCl which is easy, high yielding, and cheap as hell.
Then nitrate the shit out of it with NaNO3 or LiNO3 or HNO3, but not KNO3 (yields will be lower) to get 1,4-diamino-2,3,5,6- tetranitrobenzene. This
could then be oxidized by several oxidizing agents to HNB.[/QUOTE]
Start with p-DCB mothballs, nitrate as much as you can, then reflux with NH3, then nitrate more if necessary, then oxidise -NH2 to -NO2 with Caro's.
Totally OTC HNB 
I'm not sure if you'd be able to get 1,4-dichloro-2,3,5,6-tetranitrobenzene though, so more nitration might be necessary after the conversion of -Cl
to -NH2. But you want to nitrate it as much as possible before doing that, because it'll make the substitution of the -Cl's easier, and
p-diaminobenzene will be easy to oxidise if you try to nitrate it directly, so you'll probably end up with a high proportion of black tar, like when
you rush a TNP synth. Lots of NO2 and some black tar are the products!
|
|
|
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Nope
| Quote: | Start with p-DCB mothballs, nitrate as much as you can, then reflux with NH3, then nitrate more if necessary, then oxidise -NH2 to -NO2 with Caro's.
Totally OTC HNB [/qupte]
Wont work for the same reason that trinitration of benzene is hard.
Two electron withdrawing groups para to each other are highly deactivating. Even mononitration of p-DCB would be a difficult task, not to mention
further nitrations, where the substitutions are even more difficult because:
1.Even less electrodense ring
2.The groups present are all meta directing, but only positions open are ortho, subsitutions will be strongly discouraged, so to speak.
2-nitro-p-dichlorobenzene could only be substituted in the 5 or 6 position, with the 6 being the more likely of the two, and even that is hard.
Only one nitro group on the ring will not sufficiently weaken any ring-Cl bonds present. p-DCB is very inert because those Cl's are bound tight as
fuck to that ring.
But aniline is another story. NH2 is meta directing, I see no reason why nitration of aniline to p-nitroaniline would not be easy.
And p-diaminobenzene is extremely acitvated, and I do mean extremely. The VERY electron dense ring is vry suitable for aromatic electrophilic
substitution, definitely to the dinitro stage, likely to the trinitro stage, and maybe possible even to the tetranitro, I don't know though.
PrimoPyro |
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
In patent number 4,248,798 pentanitroaniline is produced by reducing one nitro group of TNT with H2S and then nitrating the aminodinitrotoluene with
mixed acids. A huge amount of H2SO4 is used, but only 3 mL HNO3 per gram ADNT and the product is extracted with CH2Cl2 so the sulfuric acid can
probably be recycled without much trouble. No exotic conditions are employed.
Does anyone know what the properties of pentanitroaniline are? It seems to me that it must be extremely powerful but not as unstable as HNB.
|
|
|
zodiac
Harmless

Posts: 1
Registered: 26-10-2002
Location: 121th street Phoenix
Member Is Offline
Mood: No Mood
|
|
pentanitroaniline
Pentanitroaniline ?
Is it a stuff for HardcorePyro ?
„space for more than two“
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
As was mentioned earlier, aniline is just another name for aminobenzene so pentanitroaniline is the same as aminopentanitrobenzene.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I wonder if there's no way to get those electrons of the benzene ring in a more active state by adding a strongly electrophilic specie or something.
Also, would further nitrating the NH2 group to NH(NO2) or even N(NO2)2 be possible from pentanitroaniline?
Maybe by oxidation with K2Cr2O7 or KMnO4?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Sorry, this is a fairly pointless post in that it doesn't help us get to HNB, but I thought I'd post it to illustrate the difficulty of nitrating
benzene to TNB:
"Hepp and Lobry de Bruyn improved the process, treating 60 grams of m-dinitrobenzene with a mixture of 1 kilo of fuming sulphuric acid and 500 grams
of nitric acid (d. 1.52) for 1 day at 100*C and for 4 days at 110*C..."
Taken from COPAE. And that's the IMPROVED process!
|
|
|
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Thats why you dont use HNO3 :P
Thats why you use N2O5 as the nitrating agent. Yields are better, but there are more side reactions as well, and more fumes of course.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Ah, well yes if you can get N2O5 or those crazy nitronium salts then it could be more easy to nitrate... I was assuming OTC.
|
|
|
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Of Course
And you are right, I was only being sarcastic. N2O5 could be put to much better use than nitration, using it for this would be retarded.
I do know that trinitrating aniline will be easier than trinitrating toluene. I think that phenol is the easiest monosubstituted benzene to trinitrate
though. So if thats true, you can look at it as nitrating aniline to be between toluene and phenol in difficulty. Not bad at all.
PrimoPyro
|
|
|
IodineForLunch
Harmless

Posts: 34
Registered: 28-8-2002
Location: NJ
Member Is Offline
Mood: bored
|
|
In the TNT thread philou mentioned something about treating tetranitrotoluene with a mixture of H2SO4/H2O2/HNO3 to yeild HNB. Go find it, that may be
a good method...
David Hansen
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
I also had the idea of trimerizing 1.2-dinitro-ethene to hexanitro benzene, but then dinitroethene is unstable. So, is it possible to create some kind
of 1.2-diamino-ethene, then trimerize it to an aromatic, and then somehow oxidize the amine groups (how I don't know - someone mentioned Caro's acid).
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
potassiumcarbonmonoxide
What does this have to do with HNB you'll ask?
Well, looking at the structure it might be very useful. It can be prepared by reducing potassiumcarbonate with carbon.
The name will seem a bit odd when you look at the structure, but the formula is K6C6O6 and it can also be prepared by reacting potassium metal with
hot CO gas.
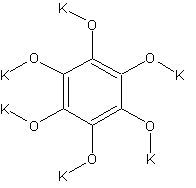
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
If you read old texts (say, Muspratt) about the carbothermic production of potassium you will be informed that this compound is a treacherous
explosive. Just warning anybody who might be about to (ha!) synthesize it.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I don't see why this would be explosive, it's merely the salt of hexahydroxybenzene.
There is however another form of potassiumcarbonmonoxide with the formula K2C2O2, K - O - C = C - O - K
("=" is triple bond here). Maybe this is the explosive one because of the acetylene bond?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
| Pages:
1
2
3 |