Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
NEST-1:A melt castable nitrate ester with high detonation performance
The Los alamos energetic material team have synthesised the denset nitrate ester known (d=1.917g/cm3). The new molecule (called NEST-1) is
a melt castable explosive (m.p=85-86°C), with performance exceeding that of HMX. The Dcj and Pcj calculated using Cheetah
thermochemical code are equal to 9.1 km/s and 40 GPa. Preliminary sensitivity test shows that NEST-1 is close to PETN in term of shock, friction and
spark stimulus. The orginal article is published in Angewandte chemie. I uploded the orginal article where they mention the synthesis.
Attachment: Synthesis of a New Energetic Nitrate Ester.zip (2MB)
This file has been downloaded 1083 times
[Edited on 3-8-2013 by Dany]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Yes , we knew. It's alright all data is welcome.
www.sciencemadness.org/talk/viewthread.php?tid=1970&page...
Rather than upload a possibly duplicated file to the forum , instead
post the link to an outside source when the file is freely available for
download. You can Google the Title of the paper to find them. Here
are two _
www.osti.gov/bridge/servlets/purl/960466-FnGuec/960466.pdf
http://permalink.lanl.gov/object/tr?what=info:lanl-repo/lare...
The file extension '.pdf ' must be added at the end of the file name to view.
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
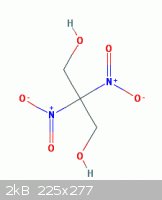
In this random post I had proposed how a related compound might be obtained
www.sciencemadness.org/talk/viewthread.php?tid=6898#pid10850...
" the possibility of obtaining 2,2-Dinitro-1,3-propanediol ( shown here below )
from nitromethane and formaldehyde see - example 4 - in the patent below
can then be further nitrated into dinitropropane dinitrate "
(CH2NO3)2=C(NO2)2 =>3 CO2 + 2 H2O + O2
https://docs.google.com/viewer?url=patentimages.storage.goog...
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Hello Franklyn,
The molecule that you proposed 2,2-dinitropropane-1,3-dinitrate) is described in the litterature.
I've done theoretical calculation on energetic properties of the tetranitrate. I estimate the condensed heat of formation based on Keshavarz empirical
method (Keshavarz, 2011). on the other hand, having the measured density (1.64 g/cm3) i calculate Dcj and Pcj based
on semi-empirical method of Kamlet & Jacobs. those are the estimated detonation performance of 2,2-dinitropropane-1,3-dinitrate:
Dcj= 8.84 km/s
Pcj= 308 kbar
condensed heat of formation: -394.53 kJ/mol
heat of detonation, Q = 1706 cal/g
OB%= +22.2
what is annoying about this compound is its low density. although, the heat of detonation is higher compared to RDX and HMX (1500 cal/g) the low
density make this compound inferior in term of detonation performance to the cyclic nitramine.
below you will find link for one patent and another article for the synthesis and reported density. also i'll post reference to Keshavarz paper for
calculating condensed heat of formation of EM and reference for estimating detonation performance of explosive.
[1] Keshavarz M.H., Prediction of the Condensed Phase Heat of Formation of Energetic Compounds, J. Hazard. Mater., 2011, 190, 330-344.
[2] Kamlet M.J., Jacobs S.J., Chemistry of Detonation. I.A Simple Method for Calculating Detonation Properties of C,H,N,O Explosives, J. Chem.
Phys., 1968, 48, 23-35.
Attachment: 2,2-dinitropropane-1,3-dinitrate US2978484A.pdf (78kB)
This file has been downloaded 634 times
Attachment: gem-Dinitro Esters. IV. Pyridine-Catalyzed Esterification of beta-Dinitro Alcohols.pdf (533kB)
This file has been downloaded 730 times
Dany.
[Edited on 10-8-2013 by Dany]
[Edited on 10-8-2013 by Dany]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
dinitropropane dinitrate seems interesting.
Its high density makes me assume it is a solid ? is it ?
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
@DubaiAmateurRocketry
before asking you should read! if you read the patent that i posted above your comment (2,2-dinitropropane-1,3-dinitrate US2978484A.pdf) you will
notice that 2,2-dinitropropane-1,3-dinitrate is a colorless mobile liquid. However, high density does not mean in necessarily that the compound is
solid.
Dany.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Dany  | @DubaiAmateurRocketry
before asking you should read! if you read the patent that i posted above your comment (2,2-dinitropropane-1,3-dinitrate US2978484A.pdf) you will
notice that 2,2-dinitropropane-1,3-dinitrate is a colorless mobile liquid. However, high density does not mean in necessarily that the compound is
solid.
Dany.
|
Ops sorry I didnt see that in the paper. what about its sensitivity ? If it is not too sensitive, then I think it would make a good plasticizer ?
and in the paper the density(1.57) is lower than what you have found on (Keshavarz, 2011) which says 1.64 ?
[Edited on 6-11-2013 by DubaiAmateurRocketry]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
@DubaiAmateurRocketry
for the second time, you should read carefully the comment before any discussion. I used the Keshavarz paper (Keshavarz, 2011) for the estimation of
heat of formation of 2,2-dinitropropane-1,3-dinitrate and not the density. The density of 1.64 g/cm3 is the experimental one reported in
the second attachement (gem-Dinitro Esters. IV. Pyridine-Catalyzed Esterification of beta-Dinitro Alcohols.pdf) which is the density measured @
25°C. In the patent (2,2-dinitropropane-1,3-dinitrate US2978484A.pdf) The reported density is 1.57 g/cm3 which has been taken @
31.5°C. So @ different temperature we should expect a density variation.
Dany.
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
I suggest reading this article for important information on improvised and conventional explosives (for J. L. Maienschein, Lawrence Livermore National
Laboratory)
https://e-reports-ext.llnl.gov/pdf/764773.pdf
Yes, it is off-topic but i don't want to open a new thread.
Dany.
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Three videos on explosions and detonation science:
1-beautiful demonstration by detonating 500 lbs of cast TNT.
http://www.youtube.com/watch?v=BWHM7HH2dkw
2-Detonation of 2000 lbs of ANFO (car bomb)
http://www.youtube.com/watch?v=3DMDF1dmgQk
3-A BBC channel report on detonation science including very informative demonstrations with explosives (1 hour report)
http://www.youtube.com/watch?v=01pjt_K-94M
Dany.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I found a picture of 5-amino-tetrazolium and TKX-50 burning finally ! All credit goes to owners.
This is 5-ATNO3.

TKX-50

Note the green flame from the TKX-50 is due to the copper plate under it. Normal should just be yellow.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Hey dany, I recently got my interest back into nitrate esters due to their amazing plasticizing effect on propellants, BTTN, EGDN, NG having amazing
plasticizing effects that are hard to replace.
we were talking about dinitropropane-dinitrate, and here, I found a extremely interesting paper researching this compound's use as a plasticizer, much
more detailed information is given.
In my opinion, this compound completely beaten NG, EGDN, and BTTN in all the important characteristics.
NPN, not only beating NG in its glass transition temperature (-81°C), it also have an OB of +12.5% and a higher density 1.66.
The only problem is that is it as sensitive as NG.
Attachment: (NPN) 2,2-Dinitro-1,3-Bis-Nitrooxy-Propane - A New Energetic Plasticizer.pdf (168kB)
This file has been downloaded 1066 times
[Edited on 23-12-2013 by DubaiAmateurRocketry]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
2-Nitropropane-1,3-dinitrate
http://journals.iucr.org/e/issues/2013/03/00/ld2094/ld2094.p...
2,2-Dinitropropane-1,3-Diol , patents
GB930385
US2522959
US3020319
US3024288
US4594430
US4774366
US4910322
http://www.chemspider.com/Chemical-Structure.68458.html
Attachment: 2,2-Dinitro-1,3-propanediol - Improved method for.pdf (137kB)
This file has been downloaded 501 times
Attachment: 2,2-Dinitropropanol Prep.pdf (410kB)
This file has been downloaded 560 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
It is quite strange they don't use the simplest synthesis of all for NEST-1...
O2N-CH2-CH2-NO2 + 4 CH2=O -mild base/H2O-> (HOCH2)2C(NO2)-C(NO2)(CH2OH)2
(HOCH2)2C(NO2)-C(NO2)(CH2OH)2 + 4 HNO3 --> (O2NOCH2)2C(NO2)-C(NO2)(CH2ONO2)2 + 4 H2O
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|