| Pages:
1
2 |
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Trichloride failure
Hey guys
Been attempting to synth some nitrogen trichloride.
I know, I know, danger. But I've been unsuccessful.
I mixed ammonia and conc. HCl to make NH4CL with a slight excess of HCl. Then added 4% NaOCl untill the entire solution went lemon yellow (this was
conducted with full protection in an ice bath). However, no oily trichloride droplets seperated out.
Any changes you could suggest to what i'm doing in order to be successful?
Thanks.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
IIRC bubbling chlorine gas through a warm solution of ammonium nitrate will work. I have never done it nor do I remember who told me but its written
in my notes to look into.
Nitrogen trichloride is very sensitive to pretty much everything. I hope you know what you're doing, it seems you might not though so be careful.
Please.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Treating everything I mix as dry NI3 don't worry.
I did try that once, didn't work for me and since then my ammonium nitrate has become to valuable to use on something that doesn't need the nitrate
ion.
My current system should work though?
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Im not sure about your method never heard anything about it, when you used the ammonium nitrate solution did you heat it?
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I didn't, that's probably a notable point. I might try it again, IIRC I kept it at icebath temperatures
I got it from the bottom of this thread http://www.sciencemadness.org/talk/viewthread.php?tid=2079
[Edited on 10-7-2013 by Tdep]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The pH has to be within a certain range, if it is too low or too high, nitrogen trichloride will not be able to form.
The optimal salt to use is ammonium sulfate (having to do with the pH), and then pass in chlorine gas.
http://books.google.com/books?id=QQkSAAAAIAAJ&pg=PA2178&...
Be sure to wear protective safety goggles! If the oily droplets start forming on the bottom of the glass detonate (which they are likely to do for no
reason), even a very small amount could shatter the glass and send fragments into your eyes.
Another strategy is to have a layer of chloroform which the NCl3 can go into as it forms. NCl3 dissolved in excess chloroform is supposedly relatively
safe to handle.
Likely reactions, if you were wondering about the chemistry of nitrogen trichloride:
NCl3 + HNO3(aq) + 3 H2O --> NH4NO3 + 3 HOCl
NCl3 + 4 HCl(aq) --> NH4Cl + 3 Cl2
NCl3 + 2 NaOH --> 2 NaOCl + NH2Cl
NCl3 + 2 NH3(aq) --> 3 NH2Cl --> further decomposition
Chlorine does not really oxidize ammonium ions. There is, however, a small equilibrium. If you want some NCl3 to form, the NH4Cl should be
very dilute.
The reason why passing chlorine gas into ammonia does not work is because chloramine initially forms, and it is not stable for long in ammonia or
alkaline solutions.
[Edited on 10-7-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
That chloroform bit is interesting, have you done it personally?
Thanks for those equations as well. Nice to see them together in one place.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
That seems a likely explanation. I believe it may have been too acidic then after the addition of the bleach, too basic.
I'll give it a shot another day with the passing chlorine through a warm solution of ammonia sulphate. That seems like a good idea (as far as NCl3
goes I guess )
I built a little bunker for the apparatus in my backyard, partly because shrapnel and partly to keep it in the dark (as I read somewhere about
detonation of it on exposure to sunlight; scary stuff!). Of course I wanted it outside due to the wonderful mix of gases you get when you violate
every warning or every bit of packaging and mix bleach, ammonia and hydochloric acid.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Chemcam, would love to see some of this stuff in action on your channel. You wouldn't be one to shy away from a challenge would you? 
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Challenge accepted. I take requests all the time and try to do most of them in a timely fashion just that the recent holiday took all my time as you
can see by the show I put on. Which by the way, I was hoping to earn a unique title dealing with pyrotechnics... 'professional pyro' would
have been nice.
But anyway yes I will make a video about this. Is there a certain way you want it done or just do it my way?
[Edited on 7-10-2013 by chemcam]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Another way to make NCl3 is electrolysis of a solution of NH4Cl using a graphite anode. At the anode, Cl2 is formed, which reacts with the ammonium
ions to NCl3. The NCl3 tends to move upwards, together with the bubbles of chlorine. In this way, you can do the experiment relatively safely, because
with electrolysis for just a few minutes you get very small amounts of chemicals.
I read that if you have a layer of unsaturated hydrocarbons (e.g. hexene) or turpentines on the solution of NH4Cl, then the bubbles of chlorine, which
have NCl3 with them in the form of small droplets lead to small flashes of light when they mix with the organic layer. The NCl3 then reacts violently
with the organic and this triggers an explosion of the small droplet. I never tried this myself though.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Would talking be too much to ask for? 
Ammonia sulphate appears to be the way to go, so a few shots of your set up would be nice. Not asking for a tutorial (hate to see very inexperienced
people try it!!) but just the real practical logistics that go behind this chemical such as how to cope without plastic, how to cope with possible
shrapnel, excess chlorine is rather interesting.
Unless you try another way and we can compare results? I plan to post a video on this, once I've got it working of course.
Also, don't die. 
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Alright I think what I will do for the video is a collaboration of all these mentioned ways to do it, electrolysis included with even this violent
reaction with an organic layer. I don't have hexene but possibly some old unknown purity turpentine that I shouldn't use probably. What else could
work?
I am going to bed now so in the morning I'll catch up on this thread and get started experimenting for the video. If somebody wants to organize the
different methods into a list for me that would greatly help and I will give credit in the video to your channel or website or whatever is needed.
I talk in the videos lol just not certain ones, people always give me shit because I sound like a city slicker and not a nerd, but, I will do the
video well and narrate entirely.
I am very cautious though, so don't worry. If I die I'll make sure beforehand I tell someone to log on here and explain what happened to me so the
same mistake is avoided.
[Edited on 7-10-2013 by chemcam]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
This is a little topic, but we all know that hydrazine can be made from hypochlorite and ammonia. Some sources get more specific and state it is the
chloramine attacking the ammonia. No doubt this will not seem like any big mystery to most of you. After all, hypochlorite is an oxidizer. However,
when one starts examining the chemistry of hypochlorite and ammonia in other situations, one realizes that hypochlorite would not be able to oxidize
NH3 like this, at least not directly. So it really becomes a sort of mystery, the exact reaction mechanism.
I have a hypothesis about how NH2Cl can oxidize NH3. The reason the reaction is so facile is likely because the hydrogen being oxidized never comes
off the nitrogen! The two reactants combine to form hydrazine chloride. None of the hydrogen-nitrogen bonds break, and the nitrogen-chlorine bond is
quite weak to begin with.
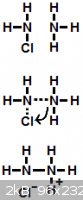
Of course, under normal conditions, the hydrazine gets further oxidized (to N2) much faster than it forms. The reason N2H4 is such a reactive reducing
agent is that the nitrogen atoms contain lone pairs that are very repulsive. N2H4 is much less stable than either NH3 or N2 because not only is the
nitrogen atom closely bonded to three other atoms, but one of those atoms also happens to be another nitrogen atom.
It is commonly stated that the NΞN triple bond is incredibly strong, but I believe that is somewhat inaccurate. The more likely reason the
formation of N2 releases so much energy is that the nitrogen atoms are more stable bonded to just one other atom so long as their valence can still be
satisfied.
In the case of NCl3, it would likely not react the same way as NH2Cl with NH3. In the case of NCl3, the nitrogen atom is more electronegative than any
one of the chlorine atoms, therefore it hydrolyzes to NH4+ ions and HOCl, rather than HNO2 and HCl. No doubt part of the reason
the central nitrogen atom is so electronegative has to do with the other two chlorine atoms it is bonded to (I am sure they are very electron
withdrawing), which alters its electronegativity.
In contrast, with NH2Cl, the chlorine atom is more electronegative than the amino group, so it is the chlorine atom that acts as the electron
acceptor. The only reason the NH2Cl can hydrolyze to ammonium ions is because it can get protonated, thus changing the electronegativity of the
central nitrogen atom. Thus, I think NH2Cl could react either way, probably depending on reaction conditions.
[Edited on 10-7-2013 by AndersHoveland]
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Well there's the electrolysis, the bubbling chlorine through an ammonia salt (or a set up more like the one here http://www.lateralscience.co.uk/oil/)
Then, I saw a while back, I have no idea if this is complete BS or not, that it can form on kids pants after they pee in the pool as the urea reacts
with the hypochlorite in the swimming pool.
To me, that's completely morbidly hilarious.
If you feel like putting your myth buster hat on i'd love to see this confirmed or denied.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes indeed. One time the door was left open to a little room under the big pool at my university. I went inside and there were two chlorine
generators, each a little bigger than a washing machine. They both had lids, and apparently they worked by adding hydrochloric acid and calcium
hypochlorite pellets. There were several big plastic barrels and buckets of these chemicals in the room. There was a big warning sign on the lids
warning not to use Triklor (TTCA), that it would cause an explosion.
I read on some other site a pool treatment expert wrote that "The oxidation of ammonia by chlorine produces about 90 % nitrogen and about 10 %
nitrate." Not sure if this is true, and if so it could just be a UV catalyzed reaction, being exposed to the sun all day.
not sure what you mean, but if you want to make chloramine gas, it is fairly simple. just mix ammonia and bleach. I am sure much of the gas that comes
out is also nitrogen, chloramine is not very chemically stable.
be warned, the reaction can be can be violent with solid hypochlorite, and chloramine gas is poisonous.
[Edited on 10-7-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This describes an insanely dangerous experiment. Do NOT repeat
this. You should use electrolysis for just a few minutes, because that produces the NCl3 in small amounts and no big blobs of oil are collected!
| Quote: | Then, I saw a while back, I have no idea if this is complete BS or not, that it can form on kids pants after they pee in the pool as the urea reacts
with the hypochlorite in the swimming pool.
To me, that's completely morbidly hilarious.
If you feel like putting your myth buster hat on i'd love to see this confirmed or denied. |
This must be
complete bullshit. Urea and hypochlorite do not react to form NCl3. They can react with formation of NH2Cl, but most of it reacts to N2. Further, any
pee would be highly diluted and the hypochlorite also is highly diluted in the swimming pool. Visible reactions of this type require at least
household bleach, without dilution!
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I think I have done a video about this a while ago. About nitrogen trichloride solution, one might be able to find it on youtube...
I never asked for this.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Oh yeah, yours is the only video there showing the chemical.
It's interesting, if a tad slow but slighty disappointed you didn't show any of its properties.
I mean, compared to the number of NI3 videos on youtube there is only one showing NCl3. I think such an interesting compound deserves a few more (even
if it is evil).
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
It was a while back, and I had hard time speaking English t that time, I wasn't very good at editing video, and my computer was/is way too slow to
properly edit them, that's why I stopped making videos.
The compound in solution slowly decompose to chlorine and nitrogen, it can dissolve various metal, and that is pretty much all what I have tested.
I never asked for this.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Don't worry, i've made muchh worse videos than that in the past. It's still very interesting and unique atm, which is hard to come by on the
youtubes now.
Is there any use for that solution of it in chloroform? Or just an interesting compound to make?
Also, I know it reacts with plastic, but here's a question: What about PTFE? Surely NCl3 wont oxidise that?
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
The title from the link really puts it in to perspective "fearless scientists with missing body parts"
I should build a robot to do this for me, maybe for now I will just bubble chlorine into an ammonium salt, since that is what I am comfortable with.
While I have the NCl3 I was thinking about this:
NCl3 + 3KBr = NBr3 + 3KCl would it work with NaBr instead of KBr?
Plante, I didn't know you had a NCl3 video, I just watched it, good job.
EDIT-
I would love to pee into a beaker of 8% bleach and I'll also find the minimum amount of urea to visibly react with that 8% bleach. (separate video
though)
[Edited on 7-10-2013 by chemcam]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<a href="http://www.lateralscience.co.uk/oil/" target="_blank"> | Quote: | <center><strong>A horrific accident in 1866 illustrates the power and sensitivity of
nitroglycerine.</strong></center>…
"In the auction room of Cobb and Sinton, on the east side of Montgomery Street, a human brain, almost intact, and other fragments of the body near it,
were found. A piece of human vertebrae was blown over the buildings on the east side of Montgomery Street, where it was picked up in front of
Squarza's, on Leidsdorff street. A piece of skull was lying on California Street, east of Leidsdorff, with other fragments of human remains, and a
human arm struck the third story window of the building across the street." <img src="../scipics/_ext.png" /> |
</a>
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Nitrogen trichloride is much more sensitive than nitroglycerin!
Nitrogen trichloride frequently explodes for no reason while it is being formed. It is so sensitive, that the detonation of a single tiny
drop is typically enough to propagate through the water and cause simultaneous explosion of all the other oily drops in the reaction container. I read
somewhere that even a tiny little spec of organic dust falling onto a drop of this oily liquid can trigger detonation.
There is absolutely no way that one could even collect a test tube of this stuff, much less store NCl3 in its pure state. If you are preparing NCl3,
you need to assume that it could explode at any moment, and handle it appropriately.
Another thing to possibly consider here is that NCl3 is supposedly a neurotoxin, so probably not a good idea to have any skin contact.
[Edited on 10-7-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
I was thinking of doing it with the chloroform layer like plante did in his video. Would that make it quite a bit safer or is that a myth?
|
|
|
| Pages:
1
2 |