| Pages:
1
2 |
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
DIY Plastic
I'm trying to find a way to cast a clear or translucent plastic at home for a project. So far I've considered bioplastic (a mix of glycerine, water,
starch and vinegar), Optix / Plexiglas(polymethyl methacrylate), and de-aerated (for lack of a better word) Styrofoam through the use of acetone
(though oddly enough, this didn't work when I tried it 5 minutes ago).
Is there any other method of creating a castable/moldable translucent plastic that anyone would like to share? I was also tempted to melt regular
plastic bottles, but the fumes and decomposition and final opacity (faster cooling = more transparency) are three things I would not much like to deal
with.
Ideas?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Sugar with minimal water can be boiled and poured to congeal into a
translucent clear material that is very brittle after it hardens. Master
desert chefs do this all the time.
Melting plastic bottles of the same material is as cheap as it gets.
just beware the risk of flaming napalm when it is melted.
Pieces of Plexiglass can be joined by wetting with methylene chloride.
A can of clear finish polyurethane varnish can be painted on a substrate
such as a plastic sheet forming a strong flexible composite. A mold form
can be repeatedly dipped and dried to achieve thickness.
Castable acrylics are available but are the most expensive solution.
.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The two simplest methods are 1) a 2 part epoxy plastic, which will work OK, especially if it is designed for that purpose (designed to be a clear
plastic) and 2) just distilling methyl methacylate from acrylic scraps (it depolymerizes upon heating) and then pouring it over the item to encase and
allowing it to sit a while (or adding a trace of a free radical initiator, eg an organic peroxide). Be warned that methyl methacylate is smelly and
toxic, so that is best done outside. It will also coat your glassware in polymeric goo, so use old glassware.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Dr.Bob  | | 2) just distilling methyl methacylate from acrylic scraps (it depolymerizes upon heating) and then pouring it over the item to encase and allowing it
to sit a while (or adding a trace of a free radical initiator, eg an organic peroxide). |
I don't quite get it - this forms a mold, or the object itself?
If I had a mold, and poured the distilled methyl methacrylate into the mold, wouldn't it harden back into the shape of the mold (making a perfect
casting)?
I hope there's some way to get rid of this gunk - otherwise this sounds promising!
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
If you do not mind using ready made plastic precursors this is very effective;
http://www.easycomposites.co.uk/Products/silicone-mould-resi...
When I was a lad these were sold as toys for teenagers but I suspect the Health and Safety elf has knocked that on the head! 
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>elementcollector1</strong>, have you considered <a href="http://en.wikipedia.org/wiki/Glyptal"
target="_blank">glyptal</a> <img src="../scipics/_wiki.png" /> resin? I thought there was a procedure for it in Pavia or Williamson,
but I can't seem to find it. Here's <a href="http://uncw.edu/chem/documents/SynthesisofPolymers.pdf" target="_blank">something</a>
<img src="../scipics/_ext.png" /> from a quick google search. I performed a similar preparation once, with good results. I used disposable
culture tubes and an open flame, rather than watch glasses. You should be able to pour and mold it while it's hot.
[edit]
Found the entry in my old lab notebook. Here's a summary:
- 0.10 g anhydrous sodium acetate + 2.00 g phthalic anhydride into each of two 15 x 85mm culture tubes
- 0.80 ml ethylene glycol and 0.80 ml glycerol added to tubes (one alcohol each) along with wooden applicator to prevent bumping/facilitate stirring
- preparations were made to collect any noxious vapors (phthalic anhydride sublimes readily)
- heated each tube with open flame until reactants fused/dissolved into homogeneous mixture
- once gas evolution (H<sub>2</sub>O) was observed, heating was continued for 5 minutes
"- after cooling, the ethylene glycol tube contained a viscous clear polymer resin
- the glycerol tube contained a clear hard resin which resembled soft amber (coloration due to the wooden stick which could no longer be
removed)—this is 'glyptal' resin
- the ethylene glycol-based resin dissolved readily in distilled H<sub>2</sub>O"
Sorry, my note-taking wasn't particularly good at the time. I did, however cite a reference in my notebook. It was indeed based upon a procedure
from Pavia.
<a href="http://books.google.com/books/about/Introduction_to_organic_laboratory_techn.html?id=SMMxAAAAMAAJ" target="_blank"><em>Procedure
36A – Polyesters</em>. <strong>Intro. to Org. Lab. Techniques: a contemporary approach</strong>. Pavia, Lampman, Kriz.
1976. Pages 282-283.</a> <img src="../scipics/_ext.png" />
[Edited on 7/9/13 by bfesser]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
This also sounds promising, as I can buy antifreeze and distill it easily.
As for the phthalic acid, that is much harder. Phthalic anhydride is apparently "A widely distributed commodity chemical", but I can't seem to find
any OTC sources for it...
EDIT: I've seen and worked with similar things as to that silicone molding package, and have a source lined up on Amazon - but I'd like to see if
there's something cheaper in the cards.
[Edited on 21-6-2013 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I purchased 500 g of phthalic anhydride from <a href="http://www.chemsavers.com/" target="_blank">Chemsavers</a> <img
src="../scipics/_ext.png" />, back when they were just an eBay seller. It had visible impurities, but that's no problem for this compound. I'm
not sure about them now, though. Perhaps someone else can advise you on this.
It's important to note that the anhydride can easily be prepared by heating the acid (loses water), the acid can easily be recrystallized from the
anhydride by boiling in water, and the anhydride is readily sublimed. So, in essence, finding either is like finding both; <em>and</em>
it's one of the simplest organics to purify, in my experience.
Note: Please see my earlier response; I've edited it.
[Edited on 7/9/13 by bfesser]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | | <strong>elementcollector1</strong>, have you considered <a href="http://en.wikipedia.org/wiki/Glyptal"
target="_blank">glyptal</a> <img src="../scipics/_wiki.png" /> resin? |
Glyptal is a trademark
for a commercial product. It's originally a contraction of "glycerol-phthalate" (or the like), as I recall. I made a post here on this material a few
years ago, but it seems to have vanishes. See this page for commercial varnishes. I believe this is the manufacturer; there are many distributors. The clear one is GLY1202. The one with red
iron oxide is GLY1201, used both as a high voltage insulator as well as a vacuum system sealant.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: fixed
broken image(s)]
[Edited on 7/9/13 by bfesser]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I guess I should mention that the point of all this was to create a peculiar shape that would 'light up' evenly from a few colored LED's - hence the
translucency and not transparency. I can get moderate results by sanding my Plexiglas, but wanted to know if there was an easier/cheaper solution.
EDIT: Something like these (actually, exactly like these).

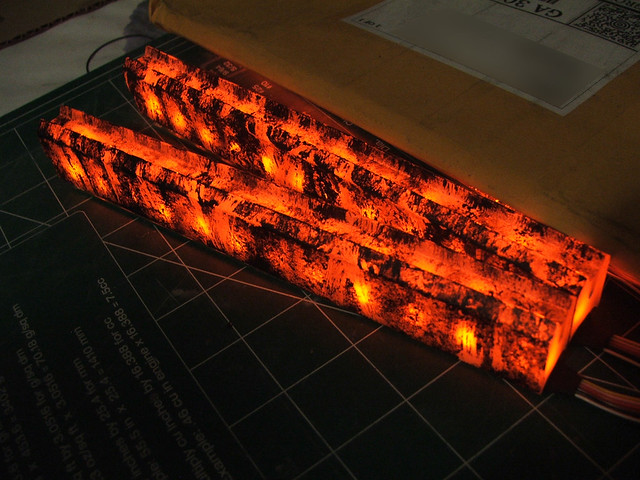
[Edited on 21-6-2013 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Hot glue. No joke; higher quality formulations are used for potting high voltage circuits.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Amazingly, that's probably the best idea I've heard all day - but how would one prevent it from sticking to the mold? It is glue, after all.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Hot glue doesn't adhere to polished surfaces. Just wait for it to cool and peel away.
You're building a <a href="https://en.wikipedia.org/wiki/Gravity_Gun#Half-Life_2_gravity_gun" target="_blank">Half-Life 2 gravity gun</a>
<img src="../scipics/_wiki.png" /> prop?
<a href="http://www.ubergizmo.com/2012/01/half-life-2-gravity-gun-prop-21000-auction/" target="_blank"><img
src="http://cdn2.ubergizmo.com/wp-content/uploads/2012/01/17-Half-Life-2-Gravity-Gun-prop.jpg" width="300" /></a> <img
src="../scipics/_ext.png" valign="top"/>
[Edited on 15.2.14 by bfesser]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Yes indeedy, and I see you know the source!
I'd have to buy a lot of hot glue to make 6"-by-1" crystals (I'm using different dimensions for mine), but at least it's OTC. Seems to work well from
my initial trials with LED's - the more glue is added on, the more diffused the light gets.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I didn't know the source. I was just intrigued by your images, and decided to investigate. Dissecting the source URL lead nowhere, so I did a <a
href="http://images.google.com/imghp?hl=en" target="_blank">Google 'search by image'</a>. Amazing what you can do on the internets
nowadays... back when I started using them, it was all Star Trek and porn…and Star Trek porn.
[Edited on 6/22/13 by bfesser]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I am recently using MDI to polymerize with some glycerin and ethylene glycol, I am worried that it says its carcinogenic. It barely have any vapor
pressure, so i think breathing near it is okay, but my hand touched it few times, but i washed them so i guess I am perfectly fine right ?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>When will you people learn to define your acronyms before just dumping them upon us‽</strong>
I assume you're referring <em>ambiguously</em> to <a href="http://en.wikipedia.org/wiki/Methylene_diphenyl_diisocyanate"
target="_blank">methylene diphenyl diisocyanate</a> <img src="../scipics/_wiki.png" /> (MDI).
[edited to remove inflammatory content]
[Edited on 7/9/13 by bfesser]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Thanks bfesser, I was kind of confused.
Quote: Originally posted by DubaiAmateurRocketry  | | I am recently using MDI to polymerize with some glycerin and ethylene glycol, I am worried that it says its carcinogenic. It barely have any vapor
pressure, so i think breathing near it is okay, but my hand touched it few times, but i washed them so i guess I am perfectly fine right ?
|
Copy-paste ninja strikes again!
| Quote: | | MDI is the least hazardous of the commonly available isocyanates but is not benign.[6] Its very low vapour pressure reduces its hazards during
handling compared to the other major isocyanates (TDI, HDI). However, it, like the other isocyanates, is an allergen and sensitizer. Persons
developing sensitivity to isocyanates may have dangerous systemic reactions to extremely small exposures, including respiratory failure. Handling MDI
requires strict engineering controls and personal protective equipment.[7] Compared to other organic cyanates, MDI has a relatively low human
toxicity. It is a potentially violently reactive material towards water and other nucleophiles. |
-
Wikipedia
Anyway, hot glue is still working out very well - now, to find a few pounds of the stuff and a wider nozzle for the glue gun.
Oh, and a few hundred more LED's.
If anyone's interested, I might post a few shots of the finished product - either in this thread, or a thread in Whimsy.
[Edited on 23-6-2013 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Variscite
Hazard to Self
 
Posts: 69
Registered: 21-5-2013
Member Is Offline
Mood: diffusing
|
|
Dubai, doing chemistry without gloves on even when working with hazardous substances? Thats not very smart. 
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Everyone starts somewhere... and some start safer than others.
Dubai, when you work with hazardous materials of any kind, it's usually a good idea to wear some gloves. I have heavy-duty yellow fake-leather
gardening gloves, and they've stood me through most of the halogens, strong mineral acids, flames, and all manner of other nasty things.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/8/13 by bfesser]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Ok I will buy some thank you.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/8/13 by bfesser]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Was the "MDI" a white crystalline solid or a yellow/ brown viscous liquid?
Also, buying just 1 pair of leather (or fake leather) gloves isn't a good solution to the problem of toxic chemicals.
Choosing suitable gloves isn't easy.
If you have a pair of gloves and spill stuff on them the stuff will, to some extent, soak into the material and diffuse through to your skin. The
only way to avoid that is to throw the gloves away before the stuff diffuses through.
In many cases (and MDI is one of them) you would be better off with thin disposable gloves.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
you might wanna consider PVC as in bottles, the bottles made with PVC is usually somewhat thick and soft'ish
the thin bottles are usually PET, poly-ethylene-terapthalate (dont know i spelled it right this time  ) )
ive melted PS on a steel plate on my hot plate and well its still sticking to the steel plate, what you need to do is melt it slowly, its very
transparent also..
usually the melting point isnt a problem, i know with PS it surely wasnt.. just low heat, but the problem is if you can actually pour this stuff
ill tell you that when you have this thing molten, and some of it is still in there, you might just wanna throw it away because its impossible to get
it off
or well i gave up on it, when small amounts were stuck to a steel plate
candle wax could possibly be done aswell but im not sure if it would be transparent enough
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by unionised  | Was the "MDI" a white crystalline solid or a yellow/ brown viscous liquid?
Also, buying just 1 pair of leather (or fake leather) gloves isn't a good solution to the problem of toxic chemicals.
Choosing suitable gloves isn't easy.
If you have a pair of gloves and spill stuff on them the stuff will, to some extent, soak into the material and diffuse through to your skin. The
only way to avoid that is to throw the gloves away before the stuff diffuses through.
In many cases (and MDI is one of them) you would be better off with thin disposable gloves. |
It is a partial transparent liquid.
color is same as when sugar is half decompose and melted, brown, a bit viscous, and no smell.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  | PVC … the thin bottles are usually PET, poly-ethylene-terapthalate (dont know i spelled it right this time  ) … PS ) … PS |
You were close.
<a href="http://en.wikipedia.org/wiki/Polyethylene_terephthalate" target="_blank">polyethylene terep<strong>h</strong>thalate
(PET)</a> <img src="../scipics/_wiki.png" />
<a href="http://en.wikipedia.org/wiki/Polyvinyl_chloride" target="_blank">polyvinyl chloride (PVC)</a> <img src="../scipics/_wiki.png"
/>
<a href="http://en.wikipedia.org/wiki/Polystyrene" target="_blank">polystyrene (PS)</a> <img src="../scipics/_wiki.png" />
[Edited on 7/9/13 by bfesser]
|
|
|
| Pages:
1
2 |