Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Peroxodicarbonates
Hello guys!
A long time ago, I've been reading about PDCs on Wikipedia. They seem as interesting, "green" oxidants. IIRC, they are prepared by very
low-temperature electrolysis of carbonate solutions.
Have anyone got any experiences with them? I think it would be difficult to make them in amateur setting because of the low temperature. Let's discuss
this. Every information is welcome.
Rest In Pieces!
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
-20 C (the temperature of the electrolysis) is quite easy to reach in a freezer. Why not just put the cell in there? Potassium carbonate is relatively
nontoxic.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Eh, and where would the transformer be connected?  I assume that one does not
want to play with f**king 9V batteries. I assume that one does not
want to play with f**king 9V batteries.
Do you know anything about its stability? Is it stable at room temp?
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It is a bit unsafe, but you could use two strips of aluminum foil, going into the refrigeration unit while the door is closed.
Or you could chill the solution before the electrolysis. With potassium carbonate dissolved in the solution, it should be able to remain unfrozen at
-15C.
I doubt the solution is going to heat up all that much during 10 minutes of electrolysis.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
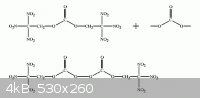
Due to the much higher density relative to liquid oxygen , compounds with
nitrocarbon functional groups such as trinitromethane contain more oxygen
by volume than liquid oxygen ! Exceeding the Oxygen Content of Liquid Oxygen
Attachment: Exceeding O2 content of LOX.pdf (170kB)
This file has been downloaded 568 times
Substituting the carbonate functional group of bis(2,2,2-trinitroethyl)carbonate
with peroxycarbonate will increase the available oxygen even more.
This would make a powerful aluminized explosive in the ratio of 2 to 1 of Al.
{-CH2(NO2)3}2:C2O6 + 8 Al => 4 Al2O3 + 6 CO + 3 N2 + 2 H2
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Quote: Originally posted by franklyn  | Due to the much higher density relative to liquid oxygen , compounds with
nitrocarbon functional groups such as trinitromethane contain more oxygen
by volume than liquid oxygen ! Exceeding the Oxygen Content of Liquid Oxygen
Substituting the carbonate functional group of bis(2,2,2-trinitroethyl)carbonate
with peroxycarbonate will increase the available oxygen even more.
This would make a powerful aluminized explosive in the ratio of 2 to 1 of Al.
{-CH2(NO2)3}2:C2O6 + 8 Al => 4 Al2O3 + 6 CO + 3 N2 + 2 H2
|
Eh, I thought we were discussing a synth that can be done in an amateur setting, and not a dangerous complex organic synth. 
This was meant to be more of a practical topic, since many people here could possibly perform the experiment.
Rest In Pieces!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Not so fast
For the HEDO-nists among us
Perchlorate and Halogen-Free High Energy Dense Oxidizers (HEDO)
www.dtic.mil/dtic/tr/fulltext/u2/a546826.pdf
Some patent literature relating to organic Peroxydicarbonates
US3377373 - Process for the Continuous Manufacture of Peroxydicarbonates *
US4359427 - Process for producing Peroxydicarbonates
US6258906 - Process for Manufacture of a Solution of Dialkyl Peroxydicarbonate
US6433208 - Method for Producing _ *
US7763690 - Method for Producing Peroxydicarbonates and _
[Edited on 12-2-2013 by franklyn]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
If I remember correctly, BTNEC was investigated as a potential energetic plasticizer. It had thermal stability problems because of the trinitromethyl
moiety. (poor thermal stability typically means it is less stable in long-term storage and gradually degrades, in some cases dangerously)
The precursor, 2,2,2-trinitroethanol is made by condensing trinitromethane with formaldehyde in the presence of a base. 2,2,2-trinitroethanol is
highly toxic, volatile, and can be absorbed through the skin.
( = extremely hazardous)
[Edited on 14-2-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|