elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Bromoskin
Just distilled some elemental bromine, and while I was drying with sulfuric acid, two drops landed on my middle finger. The splotches that immediately
resulted burned like fire, and were the usual red-brown, bromine color. I took a shower shortly afterward, and now these are almost gray (much darker
tone of skin) with a yellowish bruise in one place and a hole in the upper skin layer on the second. How serious is this, and do I need medical
attention?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
It usually disappears in 1-2 week.
Could you post a photo?
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Initially I reacted to this comment with disgust. Then I realized that a good scientist would document and report the details. It'll also serve to
deter others from carelessness with Br<sub>2</sub>. I second this. Please post a series of photographs, perhaps one every or every other
day.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
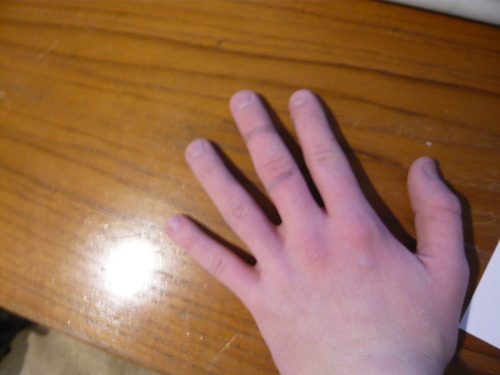
How's that? You can't really see the colored splotches, but the gray lines stand out well enough.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i had some on my fore arm last summer .... it did burn like hell thats true and felt like a bruise afterward..but it went away in a week or 10 days.
dont worry about a couple of drops on your finger, but you should ve been wearing gloves.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
That's a pity. You will recover less than 1 week(:
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Yowch! Hope you feel better soon. Were you wearing gloves?
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Gloves often don't help and sometimes even causes problems.
If wearing gloves (latex, nitrile ect.) always use a textile glove under it!
I have worked a month ago with a highly reactive pyrrole what was dissolved in THF. It accidentally spilled on my gloves and 2 hours later when I've
got off the latex glove I have noticed this on my hand:

It just polymerized on the surface of my skin and made a waterproof black layer.
It took almost a full month to get it off.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by bfesser  |
Initially I reacted to this comment with disgust. Then I realized that a good scientist would document and report the details. It'll also serve to
deter others from carelessness with Br<sub>2</sub>. I second this. Please post a series of photographs, perhaps one every or every other
day. |
Dude, where were you this summer? 
I even remember neptunium's photos of his bloddy injury. 
Documenting injuries is awesome and educational.
elementcollector1, when working with liquid bromine, always have a bucket of water nearby to plunge exposed skin into it. All it takes for gross wound
to form is few seconds of exposure.
One drop will lead to swelling of epidermal layer. It will become yellow and slough off. That's a deep second degree chemical burn.
Vapors produce first degree burns if the contact lasts long enough.
If there's a great spill on your clothes, and you don't remove them immediately, you'll get at least third degree burns.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
@neptunium and smaerd: Well, I was wearing gloves, but I took them off to ampoule the bromine (can't work the torch with gloves on). I had a glove on
the other hand, though. Does that count? XD
@kristofvagyok: ...That is a scary sight. Makes my injuries look trivial.
@Endimion: Sound advice. I'm guessing I have the second degree burn going on, as whatever isn't gray on that spot is greenish-yellow.
It was less than a milliliter, I was just so happy to have finally gotten pure bromine that I was probably a little careless. Also, it was cold
outside, and my hands were slightly shaking. Furthermore, <insert additional excuses here>.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Update: The afflicted areas have now shifted color to a dark brown. No change other than this.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also once had bromine on my hand (just a few drops). I immediately blew with my breath to evaporate the stuff and then I treated the spot with a
solution of sodium thiosulfate, which I had prepared and set aside BEFORE I did the experiments with bromine. I only ended up with two yellow spots
and had no burning sensation at all.
My advice is to have 10 ml or so of solution of sodium thiosulfate or sodium sulfite ready to grab when you do experiments with bromine. If you work
with larger amounts of bromine then also have more solution of sodium thiosulfate around.
Another very good thing to have around is dilute NH3 (e.g. 5%). This also at once destroys bromine and if you accidently inhale some Br2-vapor, then
carefully sniffing some NH3 relieves pain. Br2 and NH3 react with each other quickly and quantitatively to N2, and NH4Br, which is harmless. No side
reactions, no toxic stuff like NH2Br.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
breathing Br2 is bad enough ...how do you "carefully" sniff ammonia?? how dilute does it need to be ?
it looks good on paper and i would agree with Woelen ,but how does one go about inhaling ammonia ?
concentrated Nitric acid also leaves similar marks on the skin althought yellowish and usually painless they disapear in 2 to 3 days..
[Edited on 17-12-2012 by neptunium]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
woelen is correct, it's even better to have a thiosulphate solution nearby. And ammonia. However, a bucket of water is always a good thing to have in
a lab.
Use 5% ammonia or perhaps lower concentration. There is a small discomfort from inhaling it in normal circumstances, whereas it's actually quite a
relief when you're irritated by bromine. Don't stuff your nose in the bottle, just inhale using mouth and nose at an appropriate, relieving rate.
BTW the yellow stuff resulting from concentrated nitric acid touching the skin are nitrated (m and p) tyrosine, tryptofan and
phenylalanine aminoacids in proteins. It's called a xanthoproteic reaction.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Water isn't good for washing off bromine from skin because the solubility is too low. You were lucky because you only had two small drops on your
skin. Washing with water won't prevent severe burns from forming if you spill a larger amount of bromine!
The best immediate help is washing with sodium thiosulfate solution. What also helps very well is washing with ethanol.
When I once spilled a little bromine on my skin and didn't have thiosulfate solution at hand, I grabbed the washing bottle with ethanol instead and
used it to rinse it off. There was no visible injury to my skin.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by garage chemist  | Water isn't good for washing off bromine from skin because the solubility is too low. You were lucky because you only had two small drops on your
skin. Washing with water won't prevent severe burns from forming if you spill a larger amount of bromine!
The best immediate help is washing with sodium thiosulfate solution. What also helps very well is washing with ethanol.
When I once spilled a little bromine on my skin and didn't have thiosulfate solution at hand, I grabbed the washing bottle with ethanol instead and
used it to rinse it off. There was no visible injury to my skin.
|
Water is perfectly fine if there's a bucket of it. Just put your hand in it and swirl around. It's not just mechanical removal. Skin likes water.
Now, a bucket of sodium thiosulphate would be the best.  
Better don't use ethanol. Bromine dissolves easier in it, and ethanol transports it deeper into the skin.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by kristofvagyok  | Gloves often don't help and sometimes even causes problems.
If wearing gloves (latex, nitrile ect.) always use a textile glove under it!
I have worked a month ago with a highly reactive pyrrole what was dissolved in THF. It accidentally spilled on my gloves and 2 hours later when I've
got off the latex glove I have noticed this on my hand:

It just polymerized on the surface of my skin and made a waterproof black layer.
It took almost a full month to get it off. |
Wow I have to imagine an absolutely waterproof hand. Having a permanent glove isn't too... comfortable. Did it hurt?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eddygp  | | Wow I have to imagine an absolutely waterproof hand. Having a permanent glove isn't too... comfortable. Did it hurt? |
Nope, it didn't hurt and didn't caused any problems, except that everyone asked that what happened(:
It just made an extra (quite resistant) layer over my skin. The luck was that it immediately polymerized on contact with my skin and didn't had time
to get absorbed.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Well, it's less brown and more an ugly red, irritated skin. I *think* that's a good sign, as the brown, brominated stuff seems to be disappearing.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
The injury's almost gone, all that's left are two holes in the skin (likely from where the bromine initially touched it). Nothing seems to be
permanent, fortunately.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|