Erbium_Iodine_Carbon
Harmless

Posts: 41
Registered: 6-8-2011
Location: ON, Canada
Member Is Offline
Mood: No Mood
|
|
Hydrogen Peroxide Concentration by Desiccant
Hello all,
My apologies if this method has already been discussed; my searches have turned up nothing.
It's very hard to get a hold of >9% hydrogen peroxide where I live, so I need a reliable method to concentrate it. I've read about and tried both
evaporation and freezing but neither of these have worked to my satisfaction.
I like the idea of evaporation but I'd like to find a way to reduce the decomposition due to heat. My idea is to place a beaker with some dilute
peroxide in a sealed container and surround it with dry CaCl2. Over time, I would expect the water in the peroxide to be sucked up by the desiccant. I
would replace (and recycle) the calcium chloride once it becomes excessively wet.
Has anyone tried this? Does it work?
Any comments or suggestions are welcome.
Thanks!
[Edited on 27-10-2012 by Erbium_Iodine_Carbon]
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
I recall some discussions where hydrogen peroxide was 'salted out' from a dilute solution. I suspect your desiccant would achieve the same thing (if
it actually works).
Freezing is my preferred method for ~18% peroxide because it has been reliable and isn't energy or equipment-intensive.
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Well, I don't know if you have any near you but check/ask at health food stores. There are three stores in my city that carry 35% food grade peroxide
for about 19$/L. If they don't have any you can often have them special order it for you, no questions asked... I just mention that because I am also
in Canada and I find it pretty easy to find, just need to look in the right places 
This is the exact product that they all sell, it's made in Edmonton I believe...
http://www.goldtoporganics.com/page14/page14.html
|
|
|
Erbium_Iodine_Carbon
Harmless

Posts: 41
Registered: 6-8-2011
Location: ON, Canada
Member Is Offline
Mood: No Mood
|
|
@Mailinmypocket:
I've tried a few health food stores in my hometown but the strongest they have is 5% sold as non-chlorine bleach. I'm in Kingston now for school so I
think I'll take your suggestion and look around for it. Thanks!
@Tank:
My method would be slightly different in that the salt and peroxide would be separated, with the water in the peroxide slowly evaporating and the
calcium chloride absorbing it from the air.
I've attached 2 pictures for clarity, one showing my proposed set-up and the other a graph of percent peroxide in vapour vs. percent in solution,
showing that even at relatively high concentrations of peroxide, mostly water evaporates. I got this picture from US Peroxide who claimed to have got it from J.J. Van Laar. Z. Physik. Chem. 72:723 (1910).
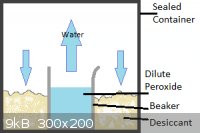 
|
|
|
plastics
Hazard to Others
  
Posts: 141
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
Here is a snippit from a paper on chemiluminescence to make high concentration H2O2 starting from 30%, no reason why it shouldn't work with a lower
concentration
Attachment: H2O2.pdf (50kB)
This file has been downloaded 821 times
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Quote: Originally posted by Erbium_Iodine_Carbon  | @Mailinmypocket:
I've tried a few health food stores in my hometown but the strongest they have is 5% sold as non-chlorine bleach. I'm in Kingston now for school so I
think I'll take your suggestion and look around for it. Thanks!
@Tank:
My method would be slightly different in that the salt and peroxide would be separated, with the water in the peroxide slowly evaporating and the
calcium chloride absorbing it from the air.
I've attached 2 pictures for clarity, one showing my proposed set-up and the other a graph of percent peroxide in vapour vs. percent in solution,
showing that even at relatively high concentrations of peroxide, mostly water evaporates. I got this picture from US Peroxide who claimed to have got it from J.J. Van Laar. Z. Physik. Chem. 72:723 (1910).
|
I would imagine that it would decompose in the process. That is the main reason why you just can't 'boil it down' as well.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
paw_20
Harmless

Posts: 32
Registered: 14-8-2012
Location: United States
Member Is Offline
Mood: Curious
|
|
Surely you would lose some to inescapable fact that H2O2 decomposes over time, but I see no reason why it would decompose any faster than a solution
of peroxide left alone. You're doing nothing to increase the energy of the system, which is why you can't boil it down. It should still be more
concentrated that what you started with.
I would however use all the usual precautions for peroxides; make sure that the container in which you are dessicating is protected from light, and
also don't actually seal it, in case there is significant decomposition (or keep it under vacuum).
plastics, did the paper say to what concentration H2O2 they were concentrating that 30% solution?
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Hydrogen Peroxide can be dehydrated by dessicants, there are a number of papers on it, the best one being either H2SO4 or
Magnesium Chlorate - Mg(ClO4)2. There are numerous dessicants that could be used, but vacuum would be an essential component
given the difference in vapor pressure.
Attachment: Maas.Hatcher.The.Properties.of.Pure.Hydrogen.Peroxide.I.pdf.I (1.6MB)
This file has been downloaded 783 times
Attachment: Maas.Hatcher.The.Properties.of.Pure.Hydrogen.Peroxide.II.pdf (120kB)
This file has been downloaded 1192 times
Attachment: Maas.Hatcher.The.Properties.of.Pure.Hydrogen.Peroxide.III.pdf (536kB)
This file has been downloaded 468 times
Attachment: Kilpatrick.Reiff.Rice.The.Preparation.of.Hydrogen.Peroxide.pdf (198kB)
This file has been downloaded 611 times
Attachment: Titova.etal.Method.for.Concentration.of.Hydrogen.Peroxide.to.Obtain.it.in.Anhydrous.Form.pdf (37kB)
This file has been downloaded 807 times
[Edited on 6-6-2013 by aliced25]
From a Knight of the Realm: "Animated movies are not just for kids, they're also for adults who do a lot of drugs." Sir Paul McCartney
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Mg(ClO4)2 is magnesium PERchlorate
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
As far as evaporation goes. The trick is to keep the temp around 180-190F and not higher. Mag stir to prevent hot spots, and bubble some dried air
into the solution from a tank pump. I imagine propping up a blow dryer pointed at the surface of the solution whilst heating gently on the hotplate
would speed it up wonderfully. I can obtain peroxide very close to 30% with this method in just a few hours. I cant seem to get this concentration
with the fractional freezing method.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|