MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Green Smash-Glow Crystals
Somewhat recently, I received the materials to try out NurdRage's "Smash Glow Crystals" experiment detailed here: http://www.instructables.com/id/Make-SMASH-GLOW-Crystals/
-----------------------------
For reference, the original procedure (summarized) to make orange smash-glow crystals is as follows:
100mL Dry Ethanol
2.93g Dibenzoylmethane
1.4g Europium(III) Nitrate
1.9mL Triethylamine
Combine all together in a flask and gently heat until everything dissolves. Let cool as slowly as possible to form crystals of the product, Europium
Tetrakis (Dibenzoylmethide)Triethylammonium. Vacuum filter the crystals, rinse with a few portions of ethanol, and allow to dry. The crystals will be
brightly fluorescent and triboluminescent, both of an orange color.
-----------------------------
I have successfully made a few crops of beautifully fluorescent and triboluminescent crystals following his process, and after doing this I had an
idea: what if I substituted terbium nitrate for europium nitrate? Terbium has green fluorescence rather than europium's orange, so my goal was to
produce green triboluminescent crystals in the end. Both elements are very similar chemically (as are most of the rare earths), and my thought was
that I could substitute Tb(NO3)3 to get the different color effect.
I followed NurdRage's procedure again, except substituting 1.48g of Tb(NO3)3 for the 1.4g Eu(NO3)3 originally called for (to account for Tb's slightly
greater atomic weight). My Tb(NO3)3 was home-made from nitric acid (also home-made) and terbium metal, the synthesis of which is detailed in my video
seen here: http://www.youtube.com/watch?v=VGLPRTH8wok
Unfortunately, it looks like my idea did not work  I was able to produce very nice
crystals of what I expect are Terbium Tetrakis (Dibenzoylmethide)Triethylammonium, which have a green tint to them as opposed to the Europium
variety's pale yellow. These crystals are somewhat larger and less "flaky" than the Eu-crystals. However, they do not fluoresce at all and show no
triboluminescence when crushed. Does anyone have any ideas as to why my idea did not work? I was pretty optimistic that it would turn out, since the
two rare earth salts are so similar chemically. The synthesis was very simple and ran just like the Eu-smash glow. The one difference I did note was
that when adding my Tb-nitrate to the mixture, a white fog was produced that looked almost identical to the fog of NH4Cl seen when combining HCl and
NH3 gases. Perhaps my terbium nitrate had some leftover nitric acid in it that fouled up the smash glow chemistry? Any thoughts are very welcome. I was able to produce very nice
crystals of what I expect are Terbium Tetrakis (Dibenzoylmethide)Triethylammonium, which have a green tint to them as opposed to the Europium
variety's pale yellow. These crystals are somewhat larger and less "flaky" than the Eu-crystals. However, they do not fluoresce at all and show no
triboluminescence when crushed. Does anyone have any ideas as to why my idea did not work? I was pretty optimistic that it would turn out, since the
two rare earth salts are so similar chemically. The synthesis was very simple and ran just like the Eu-smash glow. The one difference I did note was
that when adding my Tb-nitrate to the mixture, a white fog was produced that looked almost identical to the fog of NH4Cl seen when combining HCl and
NH3 gases. Perhaps my terbium nitrate had some leftover nitric acid in it that fouled up the smash glow chemistry? Any thoughts are very welcome.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | | The one difference I did note was that when adding my Tb-nitrate to the mixture, a white fog was produced that looked almost identical to the fog of
NH4Cl seen when combining HCl and NH3 gases. Perhaps my terbium nitrate had some leftover nitric acid in it that fouled up the smash glow chemistry?
Any thoughts are very welcome. |
Right, some nitric acid should have felt in the Tb-nitrate. Next time store it in a vacuum exsiccator over KOH pellets for a few day.
The triboluminescense is a hard topic. A lot thing varies that will a crystal produce that effect or not. Uranyl-nitrate is also triboluminescent
(gonna try it out soon ), but it is not sure that neptiunium nitrate is also...
So if it worked with Eu, it is not sure that Tb will also do it. ), but it is not sure that neptiunium nitrate is also...
So if it worked with Eu, it is not sure that Tb will also do it.
There is a simple and cheap way of making triboluminescent crytsals: not widely known but sugar (sucrose) is also triboluminescent, the only
difference is that it emits light when it breaks in the UV region, so it is not visible to naked eye. BUT, if you dope the sugar crystals with rare
earths or any UV active dye that the UV produced from the sucrose will cause the fluorescent dyes to glow, easy and cheap
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kristofvagyok  |
Right, some nitric acid should have felt in the Tb-nitrate. Next time store it in a vacuum exsiccator over KOH pellets for a few day.
|
That's actually what I did. As seen in the video, I couldn't get the Tb-nitrate to crystallize by heating so I placed it in a desiccator bag with KOH
flakes to remove the remaining water (except for water of crystallization I'm sure).
That's an interesting idea about sucrose, I hadn't heard of that. Good luck with your uranyl nitrate!
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Sugar can be visibly triboluminescent, as can adhesive tape;
http://en.wikipedia.org/wiki/Triboluminescence
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Terbium was a good idea. 
It will work with other chemicals.
Use my method! Besides Terbium you will need potassium iodide and "phenazone" which can be found in some migraine pills:
http://www.versuchschemie.de/topic,16805,-gr%FCne+Tribolumin...
Video: http://www.dailymotion.com/video/xu3f3p_tribolumineszenz-ter...
Brighness of sugar is about 1 on a scale from 0-10.
The Eu-complex is 9, the green Tb-complex is 8. The Tb-complex was the brightest compound until the Eu-complex was developed in 1966. 
Another almost equally bright compound is manganese-II-bromide triphenyphosphine oxide. This can be made with this description: http://www.chem-page.de/showexperimente/789-tribolumineszenz...
You will need MnBr2, Triophenylphosphine oxide and ethanol.
Much cheaper than Terbium.
[Edited on 23-10-2012 by Pok]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
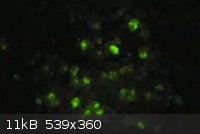
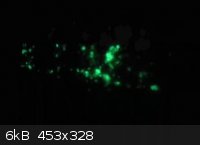
At the request of Brain&Force, here's a photo of my (presumed) terbium tetrakis(dibenzoylmethide)triethylammonium crystals. They appear slightly
more green in person. This was taken under long-tube fluorescents, as it shows the green tint the best.

|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Thanks MrHomeScientist! Would you mind if I used this photo, with credit to you, on a science fair display board? Does it show different colors in
different light sources, like Nd compounds?
I have the photo of my double sulfate on the terbium compounds thread. It was taken in regular sunlight.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Yep go ahead and use it. Sounds like a winning project there! Unfortunately no, it doesn't change colors under different lighting conditions. It only
looks very very slightly less/more yellow/green.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Does it exhibit fluorescence and phosphorescence on UV excitation? (If so, photos would be wonderful.)
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Unfortunately no, my compound does not glow at all when exposed to a black light. A real shame, because everything else in the procedure went
perfectly. The Tb crystals even have a similar shape to the Eu ones!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Is the complex magnetic? I finally got some pure Tb-sulfate and it's pretty magnetic (enough to be lifted very easily by a neodymium magnet).
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I just tried it and no, my complex is not magnetic at all. That's interesting that yours is. I had heard dysprosium salts were, but didn't know about
terbium.
|
|
|
warteo
Harmless

Posts: 42
Registered: 30-3-2011
Location: Oz
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | Unfortunately, it looks like my idea did not work  I was able to produce very nice
crystals of what I expect are Terbium Tetrakis (Dibenzoylmethide)Triethylammonium, which have a green tint to them as opposed to the Europium
variety's pale yellow. These crystals are somewhat larger and less "flaky" than the Eu-crystals. However, they do not fluoresce at all and show no
triboluminescence when crushed. Does anyone have any ideas as to why my idea did not work? I was pretty optimistic that it would turn out, since the
two rare earth salts are so similar chemically. The synthesis was very simple and ran just like the Eu-smash glow. The one difference I did note was
that when adding my Tb-nitrate to the mixture, a white fog was produced that looked almost identical to the fog of NH4Cl seen when combining HCl and
NH3 gases. Perhaps my terbium nitrate had some leftover nitric acid in it that fouled up the smash glow chemistry? Any thoughts are very welcome. I was able to produce very nice
crystals of what I expect are Terbium Tetrakis (Dibenzoylmethide)Triethylammonium, which have a green tint to them as opposed to the Europium
variety's pale yellow. These crystals are somewhat larger and less "flaky" than the Eu-crystals. However, they do not fluoresce at all and show no
triboluminescence when crushed. Does anyone have any ideas as to why my idea did not work? I was pretty optimistic that it would turn out, since the
two rare earth salts are so similar chemically. The synthesis was very simple and ran just like the Eu-smash glow. The one difference I did note was
that when adding my Tb-nitrate to the mixture, a white fog was produced that looked almost identical to the fog of NH4Cl seen when combining HCl and
NH3 gases. Perhaps my terbium nitrate had some leftover nitric acid in it that fouled up the smash glow chemistry? Any thoughts are very welcome.
|
The following might help explain why your experiment did not appear to give the desired result, it would appear that the brightness of the
triboluminesence of terbium dibenzoylmethide triethylammonium is only about 0.1% of that of europium dibenzoylmethide triethylammonium. They tried
the other lanthanides too with the Samarium compound being the next brightest at only 1.8% of the brightness of the Europium compound.
Luminescent properties of lanthanide dibenzoylmethide triethylammonium compounds
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Awesome find warteo! So it appears that triboluminescence does exist in terbium tetrakis(dibenzoylmethide)triethylammonium, but it's nowhere near as
bright. I suspected this after reading something similar about hexakis(antipyrine)lanthanide iodides - in this case only the terbium salt is strongly
triboluminescent.
MrHomeScientist: Try smashing the crystals in a VERY dark room and see what happens.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|