| Pages:
1
2
3 |
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Purification of phosphorus -- treatment of WP burns
Some of you might have seen the photos I've been uploading in the past few days, but as I got really great results after all this testing, I thought
it would be best to put it here, piled up in one thread.
I haven't specified which phosphorus in the thread's title because this might be a useful place for both RP and WP, respectively.
RP is cleaned by boiling it with distilled water for at least 15 minutes and washing with boiling distilled water over the Büchner funnel until the
collected water shows almost neutral acidity. The main impurity is phosphoric acid which cakes the powder, so after this purification, you get a puffy
red powder.
Now let's go with WP. Its main impurity is RP and assorted polymeric, orange colored varieties together with some oxidizable junk unknown to me.
Purification of Laboratory Chemicals (Armarego, Chai) describes these methods:
| Quote: |
Purified by melting under dilute H2SO4- dichromate (possible carcinogen) mixture and allowed to stand for several days in the dark at room
temperature. It remains liquid, and the initial milky appearance due to insoluble, oxidisable material gradually disappears. The phosporus can then be
distilled under vacuum in the dark. Other methods include extraction with dry CS2 followed by evaporation of the solvent, or washing with 6M HNO3,
then H2O, and drying under vacuum. |
I made my sample by dry distillation of mixture of RP and pure quartz sand to avoid bumping and to make a porous bed in the retort. If one's careful
and patient, mildly orange samples can be obtained, devoid of sand particles. The traces of residue in the retort are black and I can't identify it.
However, due to the nature of the apparatus (distilling could be done with a candle; it's very dangerous and requires an experienced experimentator,
but this is not the topic of this thread) some RP will be carried over into the receiving water.
The first thing I did was to put it in a syringe full of water and to push it through a piece of cotton at the bottom of a beaker filled with hot
water. Some of the spheres that come out are orange, some are yellow. They're all quite opaque. My conclusion is that, theoretically, proper filtering
could be enough to get very pure yellow samples, but it's simply too hard to do, so this is for removing the worst impurities. This is the filtered
product.
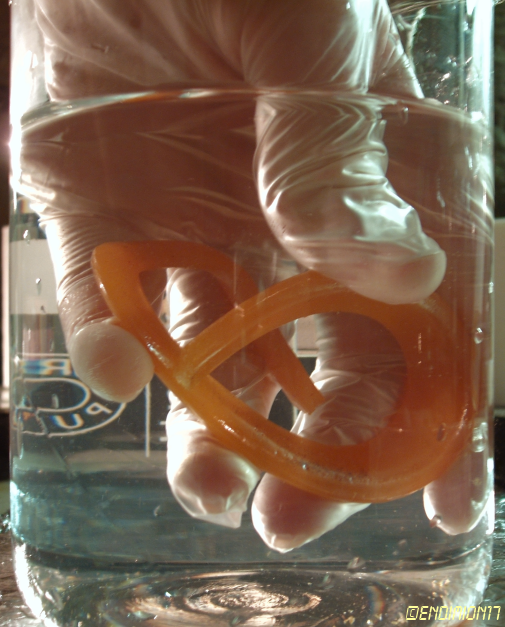
It glows, but not furiously as cleaner samples.
I've made it in the shape of a ring and, at ~ 25 °C, it behaves like a piece of plastic. It can be bent in one direction, but snaps without warning
if you try to bend it in the original position.
Next thing I did was to divide the sample into two batches. One for nitric, and one for chromic acid bath. I'll describe what I've did, and write a
recommendation.
- procedure -
I took 50 ml of distilled water, added to it 1 ml of concentrated sulphuric acid and about two tiny spatulas of potassium dichromate. I dunked the WP
inside, melted it and swirled it a couple of times. I left it at current room temperature (~28 °C), covered with a box to protect it from the light.
It remained molten.
At first some kind of yellowish, opaque precipitate formed on the surface of the sample, which was being slowly eaten as the hours progressed.
This is what it looked like after almost two days of soaking.

After removing the chromic acid, and short rinse with dilute sulphuric acid, this is what I've got.

Upon solidifying, it turned opaque and strongly yellow with a lemon tint. The orange tint was almost gone.
I've thought this was it, the best that chromic acid can do (I was wrong), so I've put it in a nitric acid bath for several hours, in a molten state,
swirling. The procedure did not appear to have altered its color significantly. It was still yellow, and the transparency was still low.

So that's what happened with the larger batch. I've cut it with a knife, melted it into ingots and stored under distilled water.

The smaller batch first went through a nitric acid bath which removed much of its orange color.

Then I tried to wash it with chromic acid. I was out for about six hours and the small batch was sitting molten, without any precipitate (obviously
because of the initial nitric acid bath), in the corrosive orange mixture.
Oh boy was I surprised when I got home and rinsed the chromic acid. 

This is the purest WP I've personally seen in my entire life. It's not colorless, it still has a hint of yellow, but it's almost
colorless. The opacity is negligible and due to the surface roughness. Upon cutting with a knife, it's like a piece of glass. The only purer sample I
saw was Theodore Gray's, but that was over the Web.
It glows better than the earlier impure samples.
In a couple of days, when I get a hold of professional DSLR camera, I'll make nice macro photos. This is just a prelude. 
Later I've melted all together, both lemon and colorless form (photo with the bottle).
- conclusion -
I don't know if chromic+nitric acid methods could replace the initial cotton filtering. I haven't tried it yet.
After cotton filtering, several hours of swirling in warm nitric acid is capable of destroying all of the opaque, sandy precipitate, and remove most
of the orange color. It can't remove all of the opaque impurities, indicating they aren't made of one compound.
Sitting under chromic acid is capable of removing both the color and precipitate, but should be used together with the nitric acid method, preferably
after it, because the nitric acid method allows the oxidation and fuming, therefore building up of phosphoric acid in the mixture.
The most important thing is to have lots of chromium(VI) above the sample and not to cover the entire bottom of the vessel. The sample should be small
(not larger than a thumbnail) to form a blob surrounded by the acid.
My first attempt failed because the amount of chromium(VI) was too low and the sample covered the entire bottom. There wasn't any speedy cleanup. The
lack of chromium(VI) was obvious by the dark brown color of the partially spent solution.
In the case of the smaller, already rather pure (HNO3) sample, the orange color of the chromic acid was almost untouched after the cleaning,
indicating there was an excess of chromium(VI).
I'll run the chromic acid bath with the combined batch once more, this time for more than few hours, because I want colorless ingots. Later, I'll
expose a piece of colorless sample to the sunlight during the day, to see the color change.
I must say it was quite entertaining to build up adrenaline while taking the large batch and cutting it with a knife, using a gloved hand in a dimly
lit room. The stuff really looks like some weird alchemical shit, like a true philosopher's stone. It glows, the vapor close to it glows and flows
like liquid, cool fire. You just want to stare at it because it's so enchanting, but you know there'll be trouble if you do it. It really looks
poisonous, like those glowing skull-shaped vapors above opened poison bottles in cartoons (perhaps there's a connection). I had the ventilation system
on maximum setting the whole time to suck the fumes away.
The feeling of cutting pure sample at room temperature is similar of cutting cold, not so old piece of resin. It is waxy, and sort of like a hard
clay, but it can burst into shards.
The colder it is, the more brittle it becomes and can snap, sending dangerous shards around the table, which is obviously very dangerous because
you've got few minutes at most before they catch fire. They melt under their own heat so you have to cool them with ice before catching them with
tweezers, or just pick the drop with paper tissue. If it's larger than 1 mm in diameter, expect it to burst into flames in a matter of seconds. Throw
it immediately in a copper(II) solution or on a safe surface to allow burning. It's advisable to do it during the night, because you can turn off the
light to see where they are.
While cutting, it's best to tilt the knife left-right, being careful before the final snap, which I did using wet gloved hands. Of course, you have to
do it in a rush. Occasional dunking in the ice cold water is preferred because it builds up heat.
[Edited on 2-8-12 by woelen]
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Glorious. That is truly a thing of beauty. Love it! There's something breathtaking about pure chemicals, even really really pure water... It
reminds me of something from Kary Mullis' autobiography "Dancing Naked in the Mind Field":
| Quote: | As described in Organic Synthesis, the phenylhydroxylamine turned into nitrosobenzene, which floated to the top. We filtered it out. It was brown
and oily, but we had about 100 grams. At $4 a gram, we were rich.
We could have taken it to Max, and he would have paid us and sold it for $6 a gram, but we were not going to make inferior chemicals just to get by.
We spent the remainder of the night purifying our product. When we were done, our crystals were white, with a very slight greenish cast in the early
morning sunlight. It was the prettiest nitrosobenzene the chemical business would ever see. We had lost about 20 percent, but what we had was pure.
We had made our first real chemical.
The next day we took the nitrosobenzene to Max, and had he not done conscientious things like that as a kid in the business, he would have been
shocked. He was pleased to the point of adopting us both as his children forever. Chemists get emotional about other chemists because of the
language they have in common and the burns on their hands. |
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
WOW. Really a nice piece you have there, would like to have one like this.
Did you bought the white phosphorus or you made it? If you made it, I would be really happy to know experimental data in a U2U.
Thanks!
I never asked for this.
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
SO BEAUTIFUL!!!
Great post.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
plante1999, preparation was described in the original post:
Quote: Originally posted by Endimion17  | I made my sample by dry distillation of mixture of RP and pure quartz sand to avoid bumping and to make a porous bed in the retort. If one's careful
and patient, mildly orange samples can be obtained, devoid of sand particles. The traces of residue in the retort are black and I can't identify it.
However, due to the nature of the apparatus (distilling could be done with a candle; it's very dangerous and requires an experienced experimentator,
but this is not the topic of this thread) some RP will be carried over into the receiving water.
|
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by BromicAcid  | plante1999, preparation was described in the original post:
Quote: Originally posted by Endimion17  | I made my sample by dry distillation of mixture of RP and pure quartz sand to avoid bumping and to make a porous bed in the retort. If one's careful
and patient, mildly orange samples can be obtained, devoid of sand particles. The traces of residue in the retort are black and I can't identify it.
However, due to the nature of the apparatus (distilling could be done with a candle; it's very dangerous and requires an experienced experimentator,
but this is not the topic of this thread) some RP will be carried over into the receiving water.
|
|
My mistake! I was too absorbed by the pictures!
Thanks!
I never asked for this.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Thanks, guys.
BromicAcid, that's a very cool quote. I know the feeling and I live for it. 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's very nice work, and thanks for the great pictures and write-up! I understand about the adrenaline - it's to be expected - after all you are
grappling with Dracula.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Dracula bit me. More info soon.
edit: the mechanical stirrer failed, fell into the chromic acid and launched a blob of WP, probably the size of a large ladybug, in the air. It
immediately started burning. Most of it fell in the floor, but some parts fell on my hand and chest. I saw it burning on my hand, which was fucking
scary. I waved my hand to extinguish it, and it worked.
I've immediately rushed to rinse it with tap water. It took me less than half minute to start doing it. A minute later, I took some copper sulphate
solution and put my hand in it to neutralize WP and make it visible. Now there are few brown stains.
As you can see in this photo made after the copper reaction, these are second degree burns. Barely 2 square centimeters, but that's quite a lot.
I let the copper do its thing for less than a minute, and then I washed it out to avoid hemolysis. For the past hour, I've been watching TV and
soaking my hand in weak saline water. The wound doesn't glow in the dark, there are no fumes, but there's still a very faint characteristic smell, or
perhaps I'm just too freaked out.
It was quite painful during the first three hours, upon removing the wound from saline.
I won't be putting any silver sulfadiazine because of its oily carrier compound that can help dissolve traces of WP into the skin.
Currently, I'm thinking of the poor victims of war crimes, how awful it must be when a significant area of your skin has third degree burns and you
know you'll die.
I'm concerned about the poisoning. Four hours have passed, and I don't feel bad at all, but I might go to the emergency in the morning just in case.
My only hope is the small affected area and small amount of WP that actually landed on it.
The clothes that saved my chest are ruined and smell like matches.
I'll tape the wet saline gauze to the wound and hope nothing happens.
The problem is - what to say to the doctor? That's where you guys step in. Please think of something. I can't just go there and tell what happened.
[Edited on 30-7-2012 by Endimion17]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
No advices, huh? 
The burn looks ok, only surface layer is missing, the next layer is pretty much intact. There are no "holes" or any other deeper pits which are common
in these cases, probably because I didn't let the stuff burn, but was furiously shaking it off.
Other wounds are fine, no surface layer detachments, only occasional boil.
I'm feeling fine. Absolutely no symptoms of poisoning and at least intestinal cramps should've started. I didn't go to the ER.
Before heading off to bed, I've put the WP on stirring in chromic acid again. I've secured the stirrer so that if it fails again, all it can do is to
embed itself into the melt.
It was stirring in the dark the whole night, and this is what I've found this morning around 11:00.

Obviously, the green stuff is chromium(III). WP has solidified in the form of sand.
Upon removal of the spent chromic acid, the product looks like this.

Absolutely no traces of yellow color. Pure white, cleaner than the last purest sample, this time the whole batch. Again, I've put it on a chromic acid
stirring bath (less than an hour, to see if the orange color changes again) and then I'm gonna melt it into ingots and take a photo.
I have reasons to believe this is beyond analytical grade stuff. Usually when you buy WP, it's yellow.
The only thing I'm puzzled about is whether the chromium(VI) was spent on impurities or was it oxidizing the phosphorus, too.
[Edited on 30-7-2012 by Endimion17]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
It seems chromic acid is being reduced by WP alone. It went green after like 30 minutes of swirling, so I replaced it with distilled water.
I can't seem to determine the color of the purified sample anymore. It became so pure its color is vague, and my lab lights are both incadescent and
fluorescent, so the stuff seems to have a very weak greenish or yelowish tint. Maybe it's because of my inability to distinguish such tints? I don't
know.

The melt seems to be a bit more opaque than earlier melts. I hope chromium(III) isn't responsible. Maybe I'm just wrong, because I'm now comparing a
small blob and a large batch. Maybe it's just turbidity because of the all night stirring. After all, there were problems with coalescing of the
droplets.

As you can see in these images , it's practically colorless, and the subtle white balance differences make it hard to determine the true color, no
matter how good you edit it afterwards.
As expected, pure WP strongly refracts light, and you can see the rainbow in the blob from the first post, and that's why the sandy WP looked
completely white.
I've took similar photos so you can compare the colors.
It would be the best to take a photo in the sunlight, but it would turn yellow, and I don't want that after all this trouble.
The glow, as usual, isn't appreciable at 0 °C, but soon after it warms up, it's easily visible even with the lights on. The purer the sample is, the
stronger the glowing.
Any ideas what to do next? Any photography wishes? Reaction videos?
BTW I've started to treat my wound with silver sulfadiazine. I guess that any traces of WP were oxidized during the night (the smell was gone before I
went to bed), and the only danger right now are bacteria. I feel fine and have no symptoms of poisoning.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I wishes you make phosphorus pentachloride next.
For the wound I think it is cured and wont be a problem.
I never asked for this.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Thanks for the pictures, absolutely beautiful. I hope your hand is ok.
Instead of Dracula maybe Lucifer is more appropriate, the bringer of light and the morning star
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm sorry to hear of your unfortunate accident with WP. It is good that you were so well prepared for such an accident, knowing what steps to take
and the appropriate treatment.
There are a number of such caged animals in our hobby. Some that I have grappled with include oleum, chlorosulfonic acid, acetic anhydride, KCN, as
well as WP. That I have so far not had such an accident is more a matter of luck than anything I think. Sometimes I have taken too many risks and
not been properly prepared for something going wrong. I have had some close calls.
Your burn looks ugly enough that it would make me seriously consider giving up experimenting with the caged animals. Eventually the lion and the
killer whale bite their handlers. I respect your love of chemistry - you went right back to your WP, continuing your research and taking those great
photos.
Whether or not to go to the emergency room was indeed a tough call. I would have been in the same predicament and can't say what I would have done if
that had happened to me.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dave Angel
Hazard to Others
  
Posts: 128
Registered: 22-3-2005
Location: UK
Member Is Offline
Mood: 0 K
|
|
Endimion17, I think you have seen that the pain that you have gone through to share these results has not gone unappreciated.
In spite of the obvious risks you have highlighted 1st hand (no pun intended), the pictures of your pure WP have inspired me to put it back on my
to-do list, having only a few weeks ago ruled it out as 'too dangerous'; it's just too good to not repeat.
Do keep us updated on the progress of your injury. If you're not already aware, there are plenty of advanced dressings for burn healing and scar
reduction etc. available these days - unless of course you wish to bear the scars as a testament to / reminder of your WP days!
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Very smart move with the CuSO4, you've obviously done your research. I would think, personally, given the size of the burn and depth that I wouldn't
go to the emergency room. Although once you get that though in your head I will admit that it is hard to extinguish.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Thanks for the feedback, I appreciate it.
The wound seems to be reacting to the sulfadiazine silver as predicted - the exposed layer is covered with goo. It's probably partially composed of white blood cells that rushed to the scene, then killed by the silver.
You can see some hemolysis at the top, that's probably where copper has lodged in the burned top layer. There's no inflamation, and no puss, the wound
doesn't smell at all. I think I'll post photos of the wound each day for the next week for you to see the progress. AFAIK, there are no such sets of
photos on the Web and I believe that would be quite useful for anyone out there who will get burned some day.
There are more burns, but as they're just boils (also 2nd degree but boring) or first degree and some superficial reduced copper marks, I'll skip
that.
As for the scars, I doubt there'll be any after few months. I've had a boiling water accident in the lab like two years ago and it involved a
sensitive area of the arm, today it's not even visible. I had quite a lot of burns in my life, even chemical ones, and I don't have visible scars.
Nevertheless, I'll ask my pharmacist if he has any advice.
This is the shirt. As you can see, my left teet  was almost burned, and one
piece almost landed on my thigh. was almost burned, and one
piece almost landed on my thigh.
I made another batch of raw WP few hours ago, and skipped the cotton and the nitric acid method. Straight to the chromic acid, but hot and swirling. I
want to simplify this as much as I can to provide a decent and less dangerous method for obtaining extremely pure samples.
This time, I'm carefully measuring the masses and durations, and will inform you of the yields. So far, they seem to be extremely high. My 0.1 g scale
didn't register any loss of the initial 3.5 g of RP, and the time for cleaning seems to be less than 15 minutes. I turned off the stirrer and now
waiting for the spheres to merge together.
Also, the amount of dichromate needed for batches of few grams is really ridiculous.
Here are 40 seconds of mesmerizing stirring action. I cranked up the voltage near the end, so the blob disappears.
<iframe sandbox width="420" height="315" src="http://www.youtube.com/embed/Z7ibNhABRX8" frameborder="0" allowfullscreen></iframe>
Sorry about the video quality, that's about the best I can do with my equipment at the moment.
[Edited on 31-7-2012 by Endimion17]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Excellent stuff Endimon. I have a couple of questions though:
| Quote: | I made my sample by dry distillation of mixture of RP and pure quartz sand to avoid bumping and to make a porous bed in the retort. If one's careful
and patient, mildly orange samples can be obtained, devoid of sand particles. The traces of residue in the retort are black and I can't identify it.
However, due to the nature of the apparatus (distilling could be done with a candle; it's very dangerous and requires an experienced experimentator,
but this is not the topic of this thread) some RP will be carried over into the receiving water. |
What material is your retort made from? What temperature are you distilling at? You say a candle flame can be used so this implies it is not terribly
high? Is the bumping very bad without the quartz sand?
I ask because I am interested in this process. I've been considering a vacuum distillation. "High vacuum" (say 10-25micron) should enable the
sublimation temperature to reach temperatures suitable for non-destructive use of quickfit glassware. How this will affect this reactive-sublimation I
am unsure. If I can get away with just using a heat gun then that will be a much simpler route I suspect.
Well done for documenting the cleaning procedure. Some solid quantities will be greatly appreciated.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Thanks. Retort was made out of regular borosilicate test tube.
I used a quiet, small, half oxidizing flame of a gas torch, but it could've been a Bunsen burner, too. The temperature doesn't have to be high. I've
mentioned candles because of the temperature. Of course, more RP, more heat. Better use propane-butane.
The bumping is not bad per se, but it can lead to a water surge, cracking of the retort and then you're screwed. I think Leidenfrost effect happens,
because RP, while gassing out P vapor, can slide towards the neck and cause mayhem.
You can use regular sand, just treat it with HCl first, then roasting, to decrease the amount of impurities.
Vacuum only lowers the boiling point... It doesn't affect the temperature of RP breakup. I'm not sure of the benefits. Your pump will be in a serious
trouble.
Don't use quickfit glassware like flasks etc, unless you have the means for purging out air. Larger glassware, more air, more initial RP fire, lower
yield. Also, working with larger amounts of RP... I'd be quite nervous. 3-4 g is my maximum. This is not something you can leave alone. You simply
have to be there, holding the burner, tapping the setup occasionally to avoid sudden caving of the material which leads to rapid release of the gas
and pyrophoric burps.
Packing of RP+sand should be like this.

There has to be a slope, to avoid a buildup of P vapor in the bottom. Receiving water should be around the melting point of WP, to avoid clogging.
I finished my quantitative experiment, so here are the results and the photo.
m(SiO2) = 0.6 g
m(RP) = 3.5 g
distilling time <20 min
m(raw WP) = 3.5 g
cleaning time (hot, swirling chromic acid) ~10 min
m(pure WP) = 3.3 g
The last mass would've been >3.4 g, if there wasn't WP mud on the top, that I couldn't join with the sample.
I recommend slow automatic stirring, without dispersion. It seems that very pure samples have trouble coalescing. That's OK if you want WP powder.

I think the color is partially due to chromic acid hydrosol. If the chromium goes from (VI) to (III), then it's a greenish tint. Dispersion is really
something that should be avoided while cleaning.
An interesting thing happens when you try to melt it. It goes completely transparent and soon it gets cloudy.

I have no idea what is happening here. WP melt should be transparent and highly refractive. No opacity.
All in all, my yield after the initial fire, the distillation and purification is a bit over 97%, and is consistent with other experiments. I don't
think I could go higher.
Don't disperse it or you'll have troubles with the mud and chromic acid hydrosol. Use slower stirring in warm water and do it overnight to save on
time and to use the lack of sunlight. It would be the best to allow few blobs swirling around, joining and breaking up like in a highspeed lava lamp.
Regarding the wound, there was a bit more hemolysis (maybe I hurt it during sleep), but no redness, so no inflamation. However, the gauze has stuck on
it, meaning it started drying and now proper epithelization should kick in. That's good, though posting daily photos of a piece of gauze looses its
purpose. I'll continue with small amount of Ag-sulfadiazine, and start daily disinfection of the surrounding area with ethanol. I have no symptoms of
poisoning except some small bad digestion and general feeling like shit, but that's common when you try my family's cuisine and don't have enough
sleep.
I'm starting with multivitamins, extra vitamin C and plenty of milk. Death can occur within a week or so, so I better deal with it. 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thanks alot for taking the time to document this properly. I really appreciate it, as I'm sure others here also do.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Day 3. The wound is wet again. This is after I removed the goo using a stream of tap water and after applying iodine to the surrounding area.
You can see there's new hemolysis. That concernes me, but is consistent with this type of chemical burn and initial copper(II) treatment. Copious goo is consistent with usage of Ag-sulfadiazine. The goo should be aseptic, and it does seem to be because there is no smell.
I've been reading a lot online and the possibility of death freaks me out. It says "even with minimal burned areas". I think that's legal bullshit.
Why would anyone die if there isn't any WP in the wound, and the wound is small? I don't have glucose-6-phosphate dehydrogenase deficiency or anything
like that, and no symptoms of poisoning.
It rarely hurts, but when it does, it makes a few annoying pulses and it's over. The pain is worse if I lower my hand, obviously.
I'm seriously reconsidering my decision to avoid visiting the ER. I just hope they're stupid enough to trust my little white lie about a "welding
accident". If they show to be very smart, I hope they'll respect the doctor-patient trust. However, I do believe they take notes and records in their
system. It would be great if the doctor finds the truth to be an exciting opportunity for learning. You don't encounter such injuries when you're
working in a small town in Europe. Heck, I'd be fucking grateful.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
firstly let me say that if you are in fear for your health don't let anyone tell you not to seek medical care. that said, the resource of first hand
knowledge available here about phosphorus burns is rather low (personally i feel that is a good thing) so the experience base to draw on is limited.
my experiences with phosphorus burns are minor but the wounds were deep and very painful. i tried making PCl3 on a test tube scale and there was a
point where my chlorine generator pushed too much gas and the result was liquified P4 sloshing out of the tube. the resulting fire melted the test
tube and glass tubing from the gas generator. when it dropped, it got on my hand and my pants. instinctively i patted the fire but that only spread it
so i dropped my pants, rolled them into a ball to extinguish the fire and wrapped a wet towel around my hand. i did not have a method of
neutralization handy so i had to get the area cold and peel the phosphorus off the wound. this was around a year ago and i have suffered no ill effect
to this day. my wife (nurse) says your wound looks healthy and said she would treat it as a normal wound. use a good triple antibiotic, and loose
gauze so it can breathe. re-dress the area every day or two. after 3-4 days let it breath so it can crust over an heal. otherwise, just keep it clean.
you have done a great job of caring for it thus far. the use of a good triple antibiotic will help to minimize the appearance of scar tissue.
again let me re-stress that if you are in fear for your health, do not let anyone tell you not to seek medical attention. i can only relate my own
experiences with this beast which do not cover all bases in any way. speedy recovery, man.
as far as the welding excuse, due to the size and area of the burn, it may be wise to say you accidentally laid the area down on a hot surface after
welding.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
endiminion, you should say you had an accident using a flare, flares often contain WP, chances are they will not ask too many questions. say you used
a road flare, or that you were on a boat,...
The flares I have on the boat contain WP, and at one point the company recalled them as they were not safe for use, something about them exploding.
I knew english wasn't your first language, by what I can make out on your P container, it says Fosfor, which means you can live in either:Belgium,
Holland, germany, and a few others.
which one is it?
EDIT:if you are going to say its a welding accident i personally think that you should say that flux dripped onto your hand, even though if you are
welding you should be wearing welding gloves lol.
Just go to ER, even if you tell them you got it purifying WP, what can they do? chances are WP is not illegal, and the cops will have a heck of a time
getting a warrant
[Edited on 1-8-2012 by Pyro]
all above information is intellectual property of Pyro.  |
|
|
freakinto
Harmless

Posts: 3
Registered: 7-3-2012
Member Is Offline
Mood: No Mood
|
|
You should really go to ER.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Tell the doctor that you found a piece of amber when you were in holidays at the baltic sea...you stored in under water and took it out at home when
it spontaneously took fire in your hand:
http://www.ncbi.nlm.nih.gov/pubmed/18926385
This is a credible argumentation!
Go to the doctor!
[Edited on 1-8-2012 by Pok]
|
|
|
| Pages:
1
2
3 |
|