AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Reaction between phosphorous and nitrogen?
I really do not know, but I do not think that elemental phosphorous readily reacts nitrogen directly. There seems to be very little information about
this available.
| Quote: | phosphorous nitride, P3N5
White, odorless, tasteless; decomposes into its elements in vacuum at high temperature. Insoluble in all solvents. Heating with water in a sealed tube
to 180 °C decomposes P3N5, forming H3PO4 and NH3. Oxygen affects it (ignition) only at temperatures above 600 °C.
Handbook of Preparative Inorganic Chemistry 2nd ed
|
Another source described "another form of phosphorous nitride sublimes" towards 1000 °C. The heat of formation of P3N5 was experimentally calculated
at "70.4 cals" (-43.9 kcal/mol ?).
| Quote: |
"Above 800 °C, P3N5 decomposes, evolving nitrogen: P3N5 --> 3 PN + N2
Phosphorous(III) nitride, PΞN, ... is metastable with respect to decomposition into P2 and N2, PN --> 1/2 P2 + 1/2 N2; Δ= -98 kJ ), but
at low temperatures it polymerises into a coloroless phosphorous(III) nitride. In addition to the above compounds, there are intractable
yellow to brown nitrides with high melting points and stoiciometries that lie somewhere between PN and P3N5.
"Inorganic Chemistry", Egon Wiberg, A. F. Holleman, Nils Wiberg, p737
|
None of the sources I have found make any mention to direct reaction between P4 and N2.
| Quote: |
"Thus phosphorus nitride is formed comparatively readily only when nitrogen interacts with atomic phosphorus..."
The literature data on the methods of preparation, structures, and properties of phosphorus nitrides PN, poly(phosphorus nitrides) (PN), (P3N5), ...
are surveyed and their practical applications, formed as a result of the direct binding of molecular nitrogen... are examined.
"Phosphorus Nitrides", Evgenii V Borisov and E E Nifant'ev
|
I suppose the ideal experiment would bring vaporised P4 at a high temperature in contact with nitrogen gas, then see if any PN polymer formed as a
powder. Simply trying to heat solid phosphorous with nitrogen would not work, because the nitride would potentially form a protective
coating, with a high decomposition temperature, that would inhibit further reaction.
[Edited on 25-5-2012 by AndersHoveland]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Few compounds react easily with gaseous nitrogen. Nitrogen is very inert. I can imagine that very hot phosphorus vapor can react with N2. As far as I
know, P4 decomposes to P2 at very high temperatures and this P2 might react with N2 and on cooling down this could give P/N compounds, but this is not
supported by any experience or literature, it just is speculation from my side.
I am quite sure that only very few people over here will have the equipment to do this kind of experiments. You need very high temperatures and
pressures. Combined with the extremely toxic and extremely flammable white P this is not an endeaver which I recommend. Practical setup of such an
experiment will elude most (if not all) of us and probably only can be done in a well equipped lab.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
If you want to prepare P3N5, this source details the synthesis and characterisation of the polymer. They used phosphine and ammonia gas heated to 800K (page 82) to synthesise
the substance. Combining phosphorus trichloride and ammonia also works apparently. They don't say anything about the reaction of nitrogen gas with P4,
probably because it just doesn't work.
Does anyone know if this polymer has a practical use? It seems to be quite unreactive, perhaps its inertness can be used for something.
A miscellaneous document of potential interest:
http://epub.ub.uni-muenchen.de/4008/1/schnick_4008.pdf
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Zan Divine
Hazard to Others
  
Posts: 170
Registered: 3-12-2011
Location: New York
Member Is Offline
Mood: Wishing all the worst that life has to offer to that SOB Wayne Lapierre
|
|
Not to be a smart-ass but the paper you referenced said that potential uses are solid state electrolytes and pigments.
He who makes a beast of himself gets rid of some of the pain of being a man. --HST
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I would think that P4 and N2 could be reacted together at some high temperature. Unlike the reaction between H2 and N2 to form ammonia, the polymer
product PN may have a high dissassociation/vaporisation temperature, which would presumably shift the equilibrium forward. In other words, high
pressure may not be required. The formation of NH3 only requires high pressures because at such high temperatures (beginning above around 455 °C )
NH3 has a tendancy to decompose back to its elements, whereas P3N5 apparently does not decompose until around 800 °C.
However, if direct combination of the elements were possible, one would wonder why all the methods of preparation for P3N5 use other more complicated
routes. While the formation of P3N5 from the elements is energetically favorable, it is only slightly so; not much energy is released.
Because of this, it is quite possible attempts at such a reaction would be overwhelmed by other unfavorable factors, such as Le Chatelier's principle.
The formation of the polymer phosphorous(III) nitride, PN(n) may be more energetically favorable, but this lower nitride also seems
to have a lower boiling point (disassociation into diatomic PN), which would not help favor its formation.
Quote: Originally posted by White Yeti  | They used phosphine and ammonia gas heated to 800K (page 82) to synthesise the P3N5. They don't say anything about the reaction of nitrogen gas with
P4, probably because it just doesn't work.
|
800 Kelvin is about 527 °C. Perhaps vaporised P4 could be reacted with ammonia? Or does the reaction mechanism require intermediate radicals that
only the thermal decomposition of phosphine can provide, perhaps such as the PH2· radical? It would be interesting if NH3 works but N2 does not in
the reaction, because it would be an instance where NH3 could act as an oxidizer where N2 could not.
[Edited on 26-5-2012 by AndersHoveland]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Zan Divine  |
Not to be a smart-ass but the paper you referenced said that potential uses are solid state electrolytes and pigments. |
Haha
I didn't read the paper in its entirety, i just skimmed over it and thought the info was worth sharing. Sorry about that.
"800 Kelvin is about 527 °C. Perhaps vaporised P4 could be reacted with ammonia? Or does the reaction mechanism require intermediate radicals that
only the thermal decomposition of phosphine can provide, perhaps such as the PH2· radical? It would be interesting if NH3 works but N2 does not in
the reaction, because it would be an instance where NH3 could act as an oxidizer where N2 could not."
To be honest, I'm not quite sure about the reaction mechanism. However, as I mentioned before, phosphine does not have to be used, phosphorus
chlorides (tri or penta) also work in this situation.
http://epub.ub.uni-muenchen.de/4008/1/schnick_4008.pdf (page 184)
I personally think this is the most viable approach:
3PCI3, + 5NH4Cl ----> P3N5 + 20HCl
They mention nothing about temperature, only the fact that this reaction is performed in a "thick-walled quartz-ampoule".
[Edited on 5-26-2012 by White Yeti]
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I have a feeling that reaction is not correct.
I am not sure whether there would be any reaction, or what exactly would form.
I did a quick calculation of the heats of formation for that equation, which suggests that the reaction you wrote would not be favorable.
This is not necessarily to say there would be no reaction, but that, if there is any reaction, the reaction products you have indicated are probably
wrong. Note though that my calculation assumed a 183.8 J/mol (not kilo Joules) for the P3N5, which is relatively negligible.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Perhaps try following some of the most classic nitride preparation paths that work with metals. For example, heat P2O5 in dry NH3:
3 P2O5 + 10 NH3 (g) --?--> 2 P3N5 + 15 H2O (g)
where water vapor is driven off to avoid a reverse reaction forming ammonia and H3PO4.
Now, if the above reaction does not proceed as shown, but reacts, what are the products? It would be interesting if the results where elemental
Phosphorous and Nitrogen (or NOx), which are varyingly observed when metal oxides react with ammonia.
Apparently, per Patent 3,226,222 "High nitrogen reaction products of nh3-p2o5 and process therefor"
(see http://www.freepatentsonline.com/3226222.pdf ) one can, in suitable conditions, form NO2 which reacts with excess ammonia, water and air to form
NH4NO3. The creation of some Urea is almost mentioned.
[Edited on 31-10-2012 by AJKOER]
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
probably because P + N are so closely, electronically, bretherin, rxns will be sparce. unlike sulfur, which is almost like a mini carbon.
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
Quote: Originally posted by AJKOER  | Perhaps try following some of the most classic nitride preparation paths that work with metals. For example, heat P2O5 in dry NH3:
3 P2O5 + 10 NH3 (g) --?--> 2 P3N5 + 15 H2O (g)
where water vapor is driven off to avoid a reverse reaction forming ammonia and H3PO4.
Now, if the above reaction does not proceed as shown, but reacts, what are the products? It would be interesting if the results where elemental
Phosphorous and Nitrogen (or NOx), which are varyingly observed when metal oxides react with ammonia.
Apparently, per Patent 3,226,222 "High nitrogen reaction products of nh3-p2o5 and process therefor"
(see http://www.freepatentsonline.com/3226222.pdf ) one can, in suitable conditions, form NO2 which reacts with excess ammonia, water and air to form
NH4NO3. The creation of some Urea is almost mentioned.
[Edited on 31-10-2012 by AJKOER] |
I'll have a loot at that patent. Catalysts must be involved, unless they are burning air!
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
Hoveland, if you'd register at a university for a 1 credit pass/fail basket-weaving course, you'd get library and SciFinder access and could answer
all these questions yourself.
Scifinder lists 3 papers with "P3N5 synthesis" in the title.
Glossing the abstracts, I see:
Pure α-P3N5 was synthesized by thermal condensation of [P(NH2)4]I at 825°...
P3N5 was obtained for the 1st time by reaction of (PNCl2)3 and NH4Cl between 770 and 1050 K...
N2 and P were supplied in the form of a gaseous mixt. at temps. in the reaction zone of 3000-3500°K ...
Good paper:
Phosphorus Nitride P3N5: Synthesis, Spectroscopic, and Electron Microscopic Investigations
Wolfgang Schnick, et.al.
Chem. Mater., 1996, 8 (1), pp 281–286
DOI: 10.1021/cm950385y
The Schnick paper describes the crystal structure as an infinite lattice:
In the solid a three-dimensional cross-linked network structure of corner sharing PN4 tetrahedra has been identified with 2/5 of the
nitrogen atoms bonded to three P atoms and 3/5 of the nitrogen atoms bonded to two P atoms.
All that, and it sorta looks like:
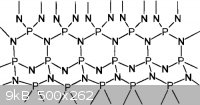
Both unreactive and nonconductive, it has been studied for use as a gate insulator in semiconductors.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by AndersHoveland  |
I did a quick calculation of the heats of formation for that equation, which suggests that the reaction you wrote would not be favorable.
This is not necessarily to say there would be no reaction, but that, if there is any reaction, the reaction products you have indicated are probably
wrong. Note though that my calculation assumed a 183.8 J/mol (not kilo Joules) for the P3N5, which is relatively negligible.
|
Notice however, that the entropy of the products is higher than that of the reactants. At a glance, it looks like the change in entropy is what drives
the reaction forward. This also compounds with the fact that the change in enthalpy is relatively small, as you pointed out.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
AndersHoveland:
With respect to Patent 3,226,222 "High nitrogen reaction products of nh3-p2o5 and process therefor", I may have misread it. There is no provided
chemical explanation of what he has created other than "chemically combined probably as amido and/or imido nitrogen with phosphorous pentoxide"
together with a demonstration that a higher nitrogen containing compound is created.
However, there may still be some validity to my comments on the chemistry of the reaction of P2O5 with NH3 in a select medium with respect to the
possible formation of NO2 (from NO and air).
[Edited on 1-11-2012 by AJKOER]
|
|
|